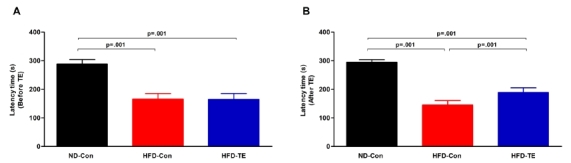
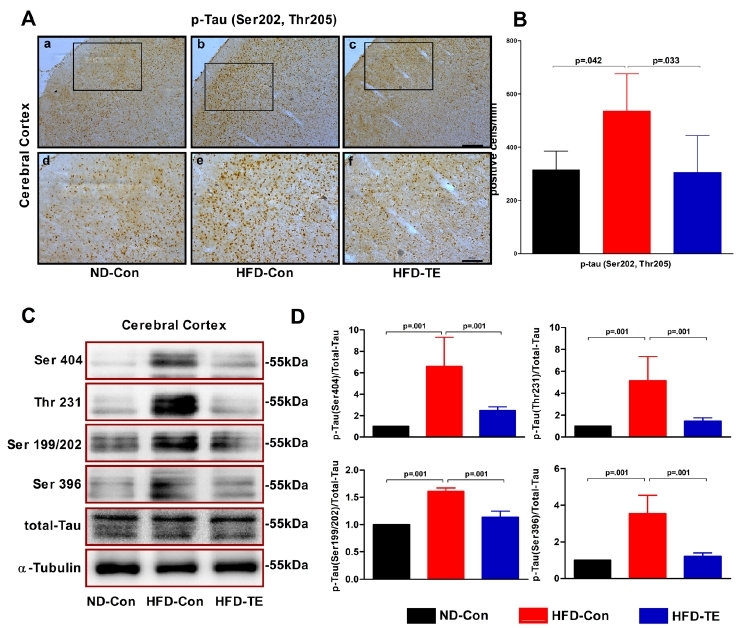
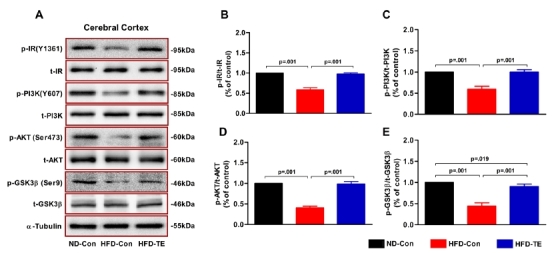
1.
Ford ES. Risks for all-cause mortality, cardiovascular disease, and diabetes associated with the metabolic syndrome: a summary of the evidence. Diabetes care. 2005;28:1769-78.

Ford ES. Risks for all-cause mortality, cardiovascular disease, and diabetes associated with the metabolic syndrome: a summary of the evidence.
Diabetes care 2005;28:1769-78. PMID:
10.2337/diacare.28.7.1769. PMID:
15983333.
2.
Volk KM, Pogrebna VV, Roberts JA, Zachry JE, Blythe SN, Toporikova N. High-Fat, High-Sugar Diet Disrupts the Preovulatory Hormone Surge and Induces Cystic Ovaries in Cycling Female Rats. J Endocr Soc. 2017;1:1488-505.


Volk KM, Pogrebna VV, Roberts JA, Zachry JE, Blythe SN, Toporikova N. High-Fat, High-Sugar Diet Disrupts the Preovulatory Hormone Surge and Induces Cystic Ovaries in Cycling Female Rats.
J Endocr Soc 2017;1:1488-505. PMID:
10.1210/js.2017-00305. PMID:
29308444.
3.
Drager LF, Togeiro SM, Polotsky VY, Lorenzi-Filho G. Obstructive sleep apnea: a cardiometabolic risk in obesity and the metabolic syndrome. J Am Coll Cardiol. 2013;62:569-76.

Drager LF, Togeiro SM, Polotsky VY, Lorenzi-Filho G. Obstructive sleep apnea: a cardiometabolic risk in obesity and the metabolic syndrome.
J Am Coll Cardiol 2013;62:569-76. PMID:
23770180.
4.
Petry NM, Barry D, Pietrzak RH, Wagner JA. Overweight and obesity are associated with psychiatric disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Psychosom Med. 2008;70:288-97.

Petry NM, Barry D, Pietrzak RH, Wagner JA. Overweight and obesity are associated with psychiatric disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions.
Psychosom Med 2008;70:288-97. PMID:
10.1097/psy.0b013e3181651651. PMID:
18378873.
5.
Tucsek Z, Toth P, Tarantini S, Sosnowska D, Gautam T, Warrington JP, Giles CB, Wren JD, Koller A, Ballabh P, Sonntag WE, Ungvari Z, Csiszar A. Aging exacerbates obesity-induced cerebromicrovascular rarefaction, neurovascular uncoupling, and cognitive decline in mice. J Gerontol A Biol Sci Med Sci. 2014;69:1339-52.


Tucsek Z, Toth P, Tarantini S, Sosnowska D, Gautam T, Warrington JP, Giles CB, Wren JD, Koller A, Ballabh P, Sonntag WE, Ungvari Z, Csiszar A. Aging exacerbates obesity-induced cerebromicrovascular rarefaction, neurovascular uncoupling, and cognitive decline in mice.
J Gerontol A Biol Sci Med Sci 2014;69:1339-52. PMID:
10.1093/gerona/glu080. PMID:
24895269.
6.
Gerozissis K. Brain insulin, energy and glucose homeostasis; genes, environment and metabolic pathologies. Eur J Pharmacol. 2008;585:38-49.

Gerozissis K. Brain insulin, energy and glucose homeostasis; genes, environment and metabolic pathologies.
Eur J Pharmacol 2008;585:38-49. PMID:
10.1016/j.ejphar.2008.01.050. PMID:
18407262.
7.
Burns JM, Donnelly JE, Anderson HS, Mayo MS, Spencer- Gardner L, Thomas G, Cronk BB, Haddad Z, Klima D, Hansen D, Brooks WM. Peripheral insulin and brain structure in early Alzheimer disease. Neurology. 2007;69:1094-104.

Burns JM, Donnelly JE, Anderson HS, Mayo MS, Spencer-Gardner L, Thomas G, Cronk BB, Haddad Z, Klima D, Hansen D, Brooks WM. Peripheral insulin and brain structure in early Alzheimer disease.
Neurology 2007;69:1094-104. PMID:
10.1212/01.wnl.0000276952.91704.af. PMID:
17846409.
8.
Steen E, Terry BM, Rivera EJ, Cannon JL, Neely TR, Tavares R, Xu XJ, Wands JR, de la Monte SM. Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer's disease--is this type 3 diabetes? J Alzheimers Dis. 2005;7:63-80.

Steen E, Terry BM, Rivera EJ, Cannon JL, Neely TR, Tavares R, Xu XJ, Wands JR, de la Monte SM. Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer's disease--is this type 3 diabetes?
J Alzheimers Dis 2005;7:63-80. PMID:
10.3233/jad-2005-7107. PMID:
15750215.
9.
Bassil F, Fernagut PO, Bezard E, Meissner WG. Insulin, IGF-1 and GLP-1 signaling in neurodegenerative disorders: targets for disease modification? Prog Neurobiol. 2014;118:1-18.

Bassil F, Fernagut PO, Bezard E, Meissner WG. Insulin, IGF-1 and GLP-1 signaling in neurodegenerative disorders: targets for disease modification?
Prog Neurobiol 2014;118:1-18. PMID:
10.1016/j.pneurobio.2014.02.005. PMID:
24582776.
10.
Ross AP, Bartness TJ, Mielke JG, Parent MB. A high fructose diet impairs spatial memory in male rats. Neurobiol Learn Mem. 2009;92:410-6.

Ross AP, Bartness TJ, Mielke JG, Parent MB. A high fructose diet impairs spatial memory in male rats.
Neurobiol Learn Mem 2009;92:410-6. PMID:
10.1016/j.nlm.2009.05.007. PMID:
19500683.
11.
Spielman LJ, Little JP, Klegeris A. Inflammation and insulin/IGF-1 resistance as the possible link between obesity and neurodegeneration. J Neuroimmunol. 2014;273:8-21.

Spielman LJ, Little JP, Klegeris A. Inflammation and insulin/IGF-1 resistance as the possible link between obesity and neurodegeneration.
J Neuroimmunol 2014;273:8-21. PMID:
10.1016/j.jneuroim.2014.06.004. PMID:
24969117.
12.
Kim YS, Morgan MJ, Choksi S, Liu ZG. TNF-induced activation

. Kim YS, Morgan MJ, Choksi S, Liu ZG. TNF-induced activation of the Nox1 NADPH oxidase and its role in the induction of necrotic cell death.
Molecular cell 2007;26:675-87. PMID:
10.1016/j.molcel.2007.04.021. PMID:
17560373.
13.
Kang EB, Koo JH, Jang YC, Yang CH, Lee Y, Cosio-Lima LM, Cho JY. Neuroprotective Effects of Endurance Exercise Against High-Fat Diet-Induced Hippocampal Neuroinflammation. J Neuroendocrinol. 2016;28.

Kang EB, Koo JH, Jang YC, Yang CH, Lee Y, Cosio-Lima LM, Cho JY. Neuroprotective Effects of Endurance Exercise Against High-Fat Diet-Induced Hippocampal Neuroinflammation.
J Neuroendocrinol 2016;28:PMID:
10.1111/jne.12385.
14.
Hetman M, Cavanaugh JE, Kimelman D, Xia Z. Role of glycogen synthase kinase-3beta in neuronal apoptosis induced by trophic withdrawal. J Neurosci. 2000;20:2567-74.

Hetman M, Cavanaugh JE, Kimelman D, Xia Z. Role of glycogen synthase kinase-3beta in neuronal apoptosis induced by trophic withdrawal.
J Neurosci 2000;20:2567-74. PMID:
10729337.
15.
Maccioni RB, Munoz JP, Barbeito L. The molecular bases of Alzheimer's disease and other neurodegenerative disorders. Arch Med Res. 2001;32:367-81.

Maccioni RB, Munoz JP, Barbeito L. The molecular bases of Alzheimer's disease and other neurodegenerative disorders.
Arch Med Res 2001;32:367-81. PMID:
10.1016/s0188-4409(01)00316-2. PMID:
11578751.
16.
Leboucher A, Laurent C, Fernandez-Gomez FJ, Burnouf S, Troquier L, Eddarkaoui S, Demeyer D, Caillierez R, Zommer N, Vallez E, Bantubungi K, Breton C, Pigny P, Bu├®e-Scherrer V, Staels B, Hamdane M, Tailleux A, Bu├®e L, Blum D. Detrimental effects of diet-induced obesity on tau pathology are independent of insulin resistance in tau transgenic mice. Diabetes. 2013;62:1681-8.

Leboucher A, Laurent C, Fernandez-Gomez FJ, Burnouf S, Troquier , Eddarkaoui S, Demeyer D, Caillierez R, Zommer N, Vallez E, Bantubungi K, Breton C, Pigny P, Bu├®e-Scherrer V, Staels B, Hamdane M, Tailleux A, Bu├®e L, Blum D. Detrimental effects of diet-induced obesity on tau pathology are independent of insulin resistance in tau transgenic mice.
Diabetes 2013;62:1681-8. PMID:
23250356.
17.
Maesako M, Uemura K, Kubota M, Kuzuya A, Sasaki K, Hayashida N, Asada-Utsugi M, Watanabe K, Uemura M, Kihara T, Takahashi R, Shimohama S, Kinoshita A. Exercise is more effective than diet control in preventing high fat diet-induced beta-amyloid deposition and memory deficit in amyloid precursor protein transgenic mice. J Biol Chem. 2012;287:23024-33.

Maesako M, Uemura K, Kubota M, Kuzuya A, Sasaki K, Hayashida N, Asada-Utsugi M, Watanabe K, Uemura M, Kihara T, Takahashi R, Shimohama S, Kinoshita A. Exercise is more effective than diet control in preventing high fat diet-induced beta-amyloid deposition and memory deficit in amyloid precursor protein transgenic mice.
J Biol Chem 2012;287:23024-33. PMID:
22563077.
18.
Takalo M, Haapasalo A, Martiskainen H, Kurkinen KM, Koivisto H, Miettinen P, Khandelwal VK, Kemppainen S, Kaminska D, M├żkinen P, Leinonen V, Pihlajam├żki J, Soininen H, Laakso M, Tanila H, Hiltunen M. High-fat diet increases tau expression in the brain of T2DM and AD mice independently of peripheral metabolic status. J Nutr Biochem. 2014;25:634-41.

Takalo M, Haapasalo A, Martiskainen H, Kurkinen KM, Koivisto H, Miettinen P, Khandelwal VK, Kemppainen S, Kaminska D, M├żkinen P, Leinonen V, Pihlajam├żki J, Soininen H, Laakso M, Tanila H, Hiltunen M. High-fat diet increases tau expression in the brain of T2DM and AD mice independently of peripheral metabolic status.
J Nutr Biochem 2014;25:634-41. PMID:
10.1016/j.jnutbio.2014.02.003. PMID:
24746833.
19.
Bradley RL, Jeon JY, Liu FF, Maratos-Flier E. Voluntary exercise improves insulin sensitivity and adipose tissue inflammation in diet-induced obese mice. Am J Physiol Endocrinol Metab. 2008;295:E586-94.

Bradley RL, Jeon JY, Liu FF, Maratos-Flier E. Voluntary exercise improves insulin sensitivity and adipose tissue inflammation in diet-induced obese mice.
Am J Physiol Endocrinol Metab 2008;295:E586-94. PMID:
10.1152/ajpendo.00309.2007. PMID:
18577694.
20.
Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol. 2011;29:415-45.

Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity.
Annu Rev Immunol 2011;29:415-45. PMID:
10.1146/annurev-immunol-031210-101322. PMID:
21219177.
21.
Zhang Y, Huang NQ, Yan F, Jin H, Zhou SY, Shi JS, Jin F. Diabetes mellitus and Alzheimer's disease: GSK-3beta as a potential link. Behav Brain Res. 2018;339:57-65.

Zhang Y, Huang NQ, Yan F, Jin H, Zhou SY, Shi JS, Jin F. Diabetes mellitus and Alzheimer's disease: GSK-3beta as a potential link.
Behav Brain Res 2018;339:57-65. PMID:
29158110.
22.
Takashima A. GSK-3 is essential in the pathogenesis of Alzheimer's disease. J Alzheimers Dis. 2006;9:309-17.

Takashima A. GSK-3 is essential in the pathogenesis of Alzheimer's disease.
J Alzheimers Dis 2006;9:309-17. PMID:
10.3233/jad-2006-9s335. PMID:
16914869.
23.
Bayod S, Del Valle J, Canudas AM, Lalanza JF, Sanchez-Roige S, Camins A, Escorihuela RM, Pall├Ās M. Longterm treadmill exercise induces neuroprotective molecular changes in rat brain. J Appl Physiol. 2011;111:1380-90.

Bayod S, Del Valle J, Canudas AM, Lalanza JF, Sanchez-Roige S, Camins A, Escorihuela RM, Pall├Ās M. Longterm treadmill exercise induces neuroprotective molecular changes in rat brain.
J Appl Physiol 2011;111:1380-90. PMID:
10.1152/japplphysiol.00425.2011. PMID:
21817108.
24.
Ohia-Nwoko O, Montazari S, Lau YS, Eriksen JL. Long-term treadmill exercise attenuates tau pathology in P301S tau transgenic mice. Mol Neurodegener. 2014;9:54.

Ohia-Nwoko O, Montazari S, Lau YS, Eriksen JL. Long-term treadmill exercise attenuates tau pathology in P301S tau transgenic mice.
Mol Neurodegener 2014;9:54PMID:
10.1186/1750-1326-9-54. PMID:
25432085.
25.
de la Monte SM, Longato L, Tong M, Wands JR. Insulin resistance and neurodegeneration: roles of obesity, type 2 diabetes mellitus and non-alcoholic steatohepatitis. Curr Opin Investig Drugs. 2009;10:1049-60.
de la Monte SM, Longato L, Tong M, Wands JR. Insulin resistance and neurodegeneration: roles of obesity, type 2 diabetes mellitus and non-alcoholic steatohepatitis.
Curr Opin Investig Drugs 2009;10:1049-60.
26.
Zhao WQ, Alkon DL. Role of insulin and insulin receptor in learning and memory. Mol Cell Endocrinol. 2001;177:125-34.

Zhao WQ, Alkon DL. Role of insulin and insulin receptor in learning and memory.
Mol Cell Endocrinol 2001;177:125-34. PMID:
10.1016/s0303-7207(01)00455-5. PMID:
11377828.