 |
 |
- Search
| Phys Act Nutr > Volume 27(1); 2023 > Article |
|
Abstract
[Purpose]
Doxorubicin (DOX) is a chemotherapeutic medication broadly used to treat diverse cancers. However, chronic DOX chemotherapy can cause myotoxicity and muscle atrophy. Endurance exercise (EXE) is used to prevent negative muscle excitation. Based on emerging evidence, this study investigated the challenges that occur in skeletal muscle quantity, quality, and metabolic determinants through autophagy, myogenic regulatory factors (MRF), antioxidant enzymes, and both the AMPK and AKT/mTOR pathways.
[Methods]
Male C57BL/6J adult mice were divided into four groups after one week of acclimation: sedentary (SED) plus saline (SAL)-receiving (SED-SAL), EXE plus SAL-receiving (EXE-SAL), SED plus DOX-receiving (SED-DOX), and EXE plus DOX-receiving (EXEDOX) groups. All mice were intraperitoneally inoculated with either SAL or DOX (5 mg/kg, every 2 weeks) for 8 weeks, while a treadmill running EXE was performed. Body weight, muscle weight, and muscle strength were measured, and the red portions of the gastrocnemius muscle were excised for biochemical analysis.
[Results]
Chronic DOX administration deteriorated body composition by decreasing body and absolute muscle weights, whereas EXE reinforced a grip strength per body weight. Although DOX inhibited BECN1 expression, EXE enhanced CS, LC3-I, LC3-II, and LAMP levels. Moreover, DOX did not interrupt MRF functions, but EXE improved MYOD without altering SOD1 or SOD2 expression. However, neither the AMPK nor the AKT/mTOR signaling pathways were associated with either DOX-receiving or EXE training.
Cancer cachexia is a multifactorial energy-wasting condition that occurs in 20% of all cancer-related death in patients with cancer [1,2]. It is characterized by a loss of body weight that includes a reduction in skeletal muscle and adipose tissue, an imbalance in the regulation of metabolism, and anorexia [3]. Although patients with cancer undergo anti-cancer chemotherapy to eliminate cancer cells, most patients suffer from crucial symptoms such as fatigue and functional disturbance of activities of daily living, specifically muscle weakness due to chronic repetitive treatment-induced myotoxicity and muscle wasting in compliance with chemotherapy cycles [4]. Thus, timely and appropriate rehabilitative therapies are essential to help counteract these side effects and prevent functional senescence (e.g., a decrease in muscular strength), and further contribute to improved quality of life with an increase in the survival rate of cancer and a decrease in mortality or morbidity to become cancer survivors [4].
Doxorubicin (DOX) is a broadly used chemotherapy drug for treating diverse types of cancer, however, the accumulation of DOX induces apoptosis in normal cells as well as in tumor cells via different pathways [5]. The precise mechanisms of DOX-induced toxicity are not fully understood, but it seems certain that the direct cytotoxic effects of DOX administration indicate two types of axes: (i) inhibition of DNA synthesis and (ii) destruction of mitochondrial respiratory function. The former occurs through the inhibition of topoisomerase-IIβ (TOP2B) and free radical-related disruption to DNA [6], whereas the latter prevails in the generation of reactive oxygen species (ROS) and causes oxidative damage to the mitochondria [7]. These biological actions predominantly appear as an etiologic phenotype in major cardiac and skeletal muscle and thereby cause myotoxicity and muscle atrophy [8,9]. In addition, DOX administration stimulates proteolytic systems (i.e., such as the ubiquitin-proteasome system (UPS), calpain/caspases, and autophagy) [10], while suppressing myogenic activity, capillary density, and antioxidant enzyme capacity [11,12].
Macroautophagy (hereafter referred to as âautophagyâ) is an elaborately controlled lysosome-dependent degradation system that maintains cellular homeostasis by removing unnecessary or dysfunctional components, including damaged proteins and mitochondria; however, dysregulation of autophagy is susceptible to muscle wasting-related diseases [13]. According to a recent systematic review, DOX activates all major protein degradation systems in skeletal muscle, and each proteolytic signaling pathway contributes to DOX-induced muscle atrophy, resulting in a decrease in the cross-sectional area (CSA) [10]. Furthermore, some studies have demonstrated that DOX inhibits protein synthesis via the mammalian target of rapamycin (mTOR), a negative regulator of autophagy [14,15].
Skeletal muscle-specific stem cells are known as satellite cells (SCs), discovered by Mauro in 1961 [16], that they play key roles in normal muscle development, conservation, self-repair, and regeneration [17]. It is sensitive to mechanical contractile activity and damaged muscle. The myogenic programming is required for the initiation of paired-box protein 7 (PAX 7) and recruitments of myogenic regulatory factors (MRF) including myogenic differentiation (MYOD) and myogenin (MYOG) by overlapping each step in a row: (i) activation (quiescence satellite to activated satellite cells) with PAX7, (ii) proliferation (activated satellite cells to myoblasts) with PAX7 and MYOD, (iii) differentiation (myoblasts to myocytes) with MYOD and MYOG, (iv) fusion (myocytes to myotubes) with MYOD and MYOG, and finally (v) maturation (myotubes to myofiber) with MYOG [17,18]. Dysregulation of MRF expression is associated with negative effects on muscle repair/regeneration in acute DOX-induced myotoxicity, but short-term endurance exercise may alleviate this effect by improving MRF expression [19,20].
AMP-activated protein kinase (AMPK) is a fundamental intracellular energy sensor that activates energy-producing catabolic signaling and inactivates energy-requiring anabolic signaling when the AMP/ATP and ADP/ATP ratios increase, leading to AMPK activation. AMPK activation is relevant to overall skeletal muscle metabolism including muscle size, protein turnover, mitochondrial oxidative capacity, and muscle integrity [21,22]. Endurance exercise training contributes to metabolic changes by reducing the negative effects of DOX chemotherapy by upregulating AMPK phosphorylation at Thr172 [14].
The AKT/mTOR pathway is a highly sensitive modulator of myofiber size that leads to changes in muscle hypertrophy and atrophy [23]. In particular, AKT (also known as protein kinase B, PKB) is a vital component of the insulin signaling pathway that plays a role in a diverse range of biological functions, such as regulation of metabolism and energy homeostasis, the activation of AKT can be estimated by phosphorylation at Thr308 by PDK1 and by phosphorylation at Ser473 by mTORC2, as a core component of mammalian target of rapamycin (mTOR) [24,25]. However, the inhibition of AKT promotes the upregulation of the forkhead box O3 (FOXO3) transcription factor and enhancement of its downstream targets, such as MuRF-1 and atrogin-1, which contribute to muscle atrophy in muscle wasting disease [26]. A recent study reported that chronic DOX treatment accelerates FOXO3-mediated catabolic signaling pathways, but prolonged endurance exercise repairs them [27].
Challenged exercise physiologists have identified the underlying cellular and molecular mechanisms by which non-pharmacological exercise (EXE) training as a safety antagonist provides a healthy skeletal muscle phenotype in impaired myocytes through in vivo studies. However, most previous studies have reported the positive effects of EXE training on skeletal muscle metabolism with limitations. Specifically, it was set by short-term (< 4 weeks) EXE training as preconditioning and even a single-bout of DOX administration [19,28-32], whereas a few studies have suggested protective effects in response to long-term EXE training (⼠4 weeks) during or after chronic DOX treatment [20,27]. However, there is still no evidence in vivo for the direct measurement of skeletal muscle functional capacities, such as grip strength, in response to chronic DOX treatment and EXE performance.
Therefore, this study examined whether long-term endurance EXE training adaptations are responsible for improving autophagy processes, MRF development, antioxidant capacity, and metabolic determinants such as the AMPK and AKT/mTOR signaling pathways, as well as skeletal muscle strength against chronic DOX chemotherapy-induced muscle wasting.
Male adult 3 months old C57BL/6J mice (DBL Company, South Korea) were utilized in this study. All mice were raised in a temperature (22ËC) and humidity (55%)-controlled facility at the Center for Laboratory Animal Sciences, under semi-specific pathogen-free (SPF) conditions with a light-dark (12:12 hrs) cycle. The mice were provided standard chow (PicoLabÂŽ Rodent Diet 20, #5053, LabDiet) and distilled water ad libitum. After one week of acclimation, the animals were randomly assigned to four groups: sedentary (SED) plus saline (SAL) treatment (SED-SAL, n=7), exercise (EXE) plus SAL treatment (EXE-SAL, n=7), SED plus DOX treatment (SED-DOX, n=7), and EXE plus DOX treatment (EXE-DOX, n=7). The body weights of these subjects were measured at the beginning and end of the experiment. All procedures were accepted by the Institute of Animal Care and Use Committee (IACUC) of Hanyang University (approval no. 2019-0039A). The mice were maintained by recommendations in the guidelines.
DOX treatment was intraperitoneally inoculated with doxorubicin hydrochloride (#D1515, Sigma-Aldrich, USA), dissolved in 0.9 % NaCl, would be applied in the same way as in a previous study [27]. DOX-treated groups were handled at a dose of 5 mg/kg, every two weeks for 8 weeks, whereas SAL-treated groups were administered with an equivalent volume of 0.9 % NaCl, normal saline (SAL) as a vehicle.
The endurance exercise training protocol was based on earlier in vivo studies that demonstrated its potency in improving muscle repair and protection against DOX-induced myotoxicity [20,27]. For a motorized treadmill adaptation, the EXE training group ran at a speed of 8-12 m/min for 30 min/day consecutively for 5 days. For progressively increased main exercise, the adapted mice performed at a speed of 12-15 m/min for 60 min/day for 8 weeks. The exercise intensity applies not until exhaustion (all-out), but a possible submaximal intensity corresponding to VO2max 70-80 % with their running speed in C57BL/6J adult mice [33]. Mice assigned to the non-EXE training groups were reserved in the same exercise training room, while those assigned to the EXE training groups were allowed to run to exclude possible confounding factors, such as the treadmillâs noise and lagging in the treadmillâs lane. In addition, some bristle brushes were set at the end of each lane without electrical shock, to minimize the distress of the mice.
A grip strength meter (#JD-A-22, JEUNGDO BIO & PLANT CO., LTD, South Korea) was utilized to evaluate the muscle strength in mice, as recorded in kilograms (kg) to the third decimal place. The apparatus included a metal bar (3 mm diameter and 10 cm length) to obtain the tensile force. A grip strength meter was placed at the bar level, and the mice were permitted to grasp the bar with only their front paws by pulling backward in the horizontal line. The forelimb grip strength examination was checked 24 h after the end of DOX administration and EXE training. The maximal force was measured twice, and higher values were determined as peak tension with the conversion of units from kilograms to grams.
DOX treatment significantly reduces the contractile power of rat skeletal muscles, particularly slow-twitch muscles, in a dose-dependent manner [34,35]. Further, gastrocnemius (GAS) muscles are mixed muscle fibers, having both red and white muscles. Importantly, in this study, only red parts of the GAS muscles were selected as representative muscles except for the white parts of the muscles to prevent a predictable confounding effect due to some different biochemical characteristics between muscle fiber types that lead to affect data values with a variety of ranges. Targeted GAS red muscles were excised, instantly frozen in liquid nitrogen, and kept at -80ËC until subsequent immunoreactive assay.
The red portions of the GAS muscles were homogenized (1:20 w/v) in T-PERÂŽ Tissue Protein Extraction Reagent (#78510, ThermoFisher Scientific) containing Halt⢠Protease and Phosphatase Inhibitor Cocktail, 100X (#78440, ThermoFisher Scientific) with a homogenizer stirrer (#HS-30E, DAIHAN Scientific) and a tissue grinder (#SL.cw.011.102, SciLabÂŽ Korea). Observing the manualâs guidelines, the homogenates were centrifuged at 10,000 Ă g for 15 min at 4ËC in a Sorvall Legend Micro 17R centrifuge (#75002444, ThermoFisher Scientific) to get the upper cytosolic fraction. The protein concentration was measured using the Pierce⢠Coomassie (Bradford) protein assay kit (#23200, ThermoFisher Scientific). The supernatants were blended into a mixture of 4X Bolt⢠LDS sample buffer (#B0008, Invitrogen) and 10X Bolt⢠sample reducing agent (#B0009, Invitrogen) at 70ËC for 15 min and cooled on ice for 15 min. Equal amounts of 40 Îźg of the protein were loaded onto Bolt⢠4-12% Bis-Tris Plus Gels, 15-well (#NW04125BOX, Invitrogen) for target proteins and Bolt⢠12% Bis-Tris Plus Gels, 15-well (#NW00125BOX, Invitrogen) for LC3B (to detect double bands and low kDa of protein) for 1 h at room temperature (RT) in diluted 20X Bolt⢠MOPS Running Buffer (#B000102, Invitrogen). After electrophoresis, the commercial gels were transferred in diluted 20X Bolt⢠Transfer Buffer (#BT00061, Invitrogen) to 0.45 Îźm nitrocellulose membranes (#1620115, Bio-Rad) or 0.2 Îźm PVDF membranes (#1620112, Bio-Rad) and used to a hydrophobic and low kDa of protein (LC3B) for 1 h at RT. To confirm the perfect transfer and equal protein loading in each lanes [14,27], the membranes were briefly stained with Ponceau S solution (#P7170, Sigma-Aldrich) and rinsed with pure water until the background disappeared. Non-specific proteins were blocked for 60 min at RT in 5 % non-fat milk or bovine serum albumin (BSA) in Tris-buffered saline containing 0.1% tween-20 (TBS-T). The membranes were then incubated with the designated primary antibodies overnight at 4ËC. The primary antibodies were used as follows: CS (#390693), PAX7 (#81975), MYOD(#377460), and MYOG (#12732) from Santa Cruz Biotechnology; AMPK(#5831), p-AMPKThr172(#2535), AKT(#9272), p-AKTThr308(#13038), p-AKTSer473(#4060), mTOR(#2983), p-mTORSer2448(#5536), BECN1(#3495), ATG7(#8558), p62(#23214), SOD1 (#37385), and SOD2 (#13142) from Cell Signaling Technology; CTSL (#133641) from Abcam; LAMP2 (#PA1-655) from ThermoFisher Scientific; LC3B(#L7543) from Sigma-Aldrich. The next day, the membranes were washed thrice for 5 min in 1X TBS-T and incubated with the designated HRP-conjugated secondary antibodies for 1 h at RT. Secondary antibodies used were goat anti-rabbit IgG (#G-21234), and goat anti-mouse IgG (#62-6520) from ThermoFisher Scientific. Next, the membranes were washed thrice for 5 min in 1X TBS-T, and immunoreactive proteins were detected using Amersham ECL western blotting detection reagent (#RPN2209, Cytiva). Band images were acquired using a ChemiDoc⢠XRS+ Imaging System (#1708265, Bio-Rad), and band intensities were quantified using Image Lab⢠software (version 6.1; #1709690, Bio-Rad). The expression levels of all proteins are presented as fold-change by percentage.
All data analyses were conducted using GraphPad PrismÂŽ software (version 9.0; San Diego, CA, USA). Values are presented as mean Âą SEM. A two-way analysis of variance was used to determine the effects of two independent variables: drug treatment and exercise performance. Tukeyâs honestly significant difference (HSD) test was used as a post-hoc test to examine where significant differences occurred. Statistical significance was set at Îą < 0.05.
The initial body weight for all groups of mice was equalized at the same level, but the final body weight for these groups was significantly different between SAL-treated and DOX-treated groups. Moreover, the absolute GAS red muscle weight was significantly decreased in the DOX-treated groups compared to the SAL-treated groups, but these phenotypes did not show relative GAS red muscle weight (Table 1). To examine whether DOX chemotherapy-induced muscle wasting affects skeletal muscle function, grip strength was measured in each group of mice (Figure 1A). The SALEXE group had the highest levels among the SED-SAL, SED-DOX, and EXE-DOX groups (Figure 1B). Exercise training reinforced a grip strength divided by body weight in the SAL-EXE and DOX-EXE groups (Figure 1C).
The effects of long-term endurance EXE training occurred in the targeted GAS red muscles by increasing CS protein levels in the EXE-DOX group compared with the SED-DOX group, whereas the EXE-SAL group showed no significant changes compared with the SED-SAL group (Figure 2A and 2B). Although there was no significant change in the response to DOX treatment, the SED-DOX group tended to have lower CS protein levels than the SEDSAL group (p=0.0533). To demonstrate the sequence of autophagy flux, each step of the vital markers was measured in a row by measuring BECN1, ATG7, p62, LC3B turnover, LAMP2, and CTSL protein levels. First, the induction of autophagy was suppressed by a decrease in BECN1 protein levels in the SED-DOX group compared with those in the SED-SAL and EXE-SAL groups. However, EXE training was increased in the EXE-SAL group compared to those in the SED-SAL and EXE-DOX groups (Figure 2A and 2C). However, neither ATG 7 nor p62 protein levels were altered in DOX-received or EXE-performed mice (Figure 2A, 2D, and 2E). As a key regulator of autophagy, LC3B turnover results in the transition from LC3B-I to LC3B-II. DOX administration was stimulated by boosting LC3B-I protein levels in the SED-DOX group compared to the SED-SAL and EXE-SAL groups, and it was most upregulated in the EXE-DOX group compared to the SED-SAL group. Moreover, the LC3B-II protein levels were significantly higher in the EXE-DOX group than in the other three groups. The LC3B II/I ratio was not significantly modified in any group, even though it appeared to rebound after EXE training (Figure 2A and 2F). To fuse with lysosomes and autolysosomes, the participation of LAMP2 and CTSL proteins is required. LAMP2 protein expression was strongly associated with both DOX-receiving and EXE-training; in particular, the DOX chemotherapy groups (SED-DOX and EXE-DOX) had higher LAMP2 protein levels than the non-DOX chemotherapy groups (SED-SAL and SED-EXE), and EXE training-induced adaptations were amplified for LAMP2 activity in the EXE-DOX group and the EXE-SAL groups (Figure 2A and 2G). In an undramatic manner, there were no alterations in either DOX-received or EXE-trained groups by a lysosomal hydrolytic protease, CTSL, which might be predictable as the efficacy of protein degradation in lysosomes (Figure 2A and 2H).
To verify whether chronic DOX treatment impaired MRF expression and thereby repaired EXE training, the biomarkers of satellite cell activation (PAX7) and myogenesis (MYOD and MYOG) were analyzed. Although PAX7 and MYOG protein expression was not affected by DOX and EXE training, only the upregulated MYOD protein levels revealed susceptibility to EXE training (Figure 3A and 3B). In addition, neither DOX administration nor EXE performance was catalyzed by antioxidant capacity at the translational levels of SOD 1 and SOD 2 proteins (Figure 3A, 3C, and 3D).
The major metabolic signaling pathways were inspected which key determinants are responsible for endurance EXE training-induced adaptations against DOX treatment-induced muscle wasting. Total AMPK was decreased in the SED-SAL and EXE-DOX groups compared to that in the SED-SAL group, and the level in the EXE-DOX group was lower than that in the EXE-SAL group. However, neither the p-AMPKThr172 nor the p-AMPKThr172/AMPK ratio was significantly changed in any of the groups (Figure 4A and 4B). AKT is phosphorylated at Thr308 by PDK1 via insulin or growth factor stimulation. Although total AKT was elevated in the SED-DOX and EXE-DOX groups compared with that in the SED-SAL group and the EXE-DOX group was greater than that in the SED-DOX group, there was no response for p-AKTThr308 activity in each group, and the p-AKTThr308/AKT ratio was not a significantly changed (Figure 4A and 4C). In addition, AKT is phosphorylated at Ser473 by mTORC2, and its activity is associated with diverse biological functions of downstream intracellular proteins, such as FOXO and mTOR [25]. Increased total AKT protein levels were observed in the SED-DOX and EXEDOX groups compared to those in the SED-SAL and EXEDOX groups, which were higher than those in the SEDDOX group (Figure 4A and 4D). The p-AKTSer473 protein levels were augmented in the SED-DOX and SED-SAL groups compared to the SED-SAL group, and even the EXE-DOX group accumulated compared to the EXE-SAL group. However, the p-AKTSer473/AKT ratio did not change significantly between all groups. Lastly, the p-mTORSer2448/mTOR ratio did not show any modifications because there were no significant changes in either p-mTORSer2448 or total mTOR protein levels in response to DOX treatment and EXE performance (Figure 4A and 4E).
This study mainly indicated that endurance exercise could be recommended as an adjuvant therapy by delaying accelerated muscle wasting and preventing declined muscle strength because of chronic DOX chemotherapy, especially considering compliance with chemotherapy rules and generating EXE training-induced adaptations through upregulation of CS protein levels. To investigate the potent cellular and molecular mechanisms of skeletal muscle regeneration and their relationships with chronic DOX-induced muscle wasting and endurance EXE training, fundamental indices (body weight, muscle weight, and muscle strength), and dominant signaling pathways (autophagy, MRF expression, antioxidant capacity, and metabolic determinants) were explored in high-affinity adapted skeletal muscle tissues.
Over the past decades, research pioneers have demonstrated that the underlying cellular and molecular mechanisms of DOX treatment have a direct effect on skeletal muscle caused by DNA damage and mitochondrial impairments, eventually leading to muscle atrophy through upregulation of free radicals, apoptosis, directly/indirectly defective mitochondria-induced ROS production, major proteolytic systems including UPS and autophagy, inflammation, antioxidant capacity, iron dysregulation, and MRF dysfunction [19,28-31,36,37]. However, unfortunately, most of the studies were conducted regardless of cancer chemotherapy rules, a single bout of acute DOX administration, and either pre-conditioning or post-conditioning of short-term exercise.
Prolonged endurance training-induced adaptations stimulate changes in enzymatic functions, metabolic reactions, and transcriptional/translational expression, which affect skeletal muscle morphology and EXE performance [38]. Thus, preserving fine-tuned remodeling through long-term exercise training can ameliorate metabolic dysfunction and prevent chronic diseases, including cancer-induced muscle wasting. For these reasons, contrary to the other studies mentioned above, this experimental study was designed with 8 weeks of endurance EXE training in parallel with chronic DOX chemotherapy.
Previous studies have suggested representative features of DOX treatment, such as myotoxicity and muscle atrophy, which are associated with decreased body weight, muscle weight, and CSA in targeted GAS muscles [14,39]. These results also identified a similar phenotype with loss of body weight and absolute muscle weight, although the relative muscle weight did not change during chronic DOX-induced muscle wasting. They did not show normalized relative muscle weights and were used in mixed fibers of the total GAS muscles without separation between the red and white fibers. Importantly, these two types were isolated, to minimize confounding factors, since DOX-induced unfavorable characteristics substantiate that the degree of dysfunction is greater in the soleus (SOL) muscles (mainly composed of slow twitch) than in the extensor digitorum longus (EDL) muscles (mainly composed of fast twitch), with a differential effect on skeletal muscle fiber types [34,35]. For this reason, it is a rationale explaining why most of the previous studies targeted SOL muscles, but it is not enough sample volume to examine to trial a variety of metabolic biomarkers. Thus, isolated GAS red muscles were selected for this study.
More importantly, this study is the first attempt to directly test skeletal muscle function in response to both chronic DOX-receiving and EXE training using a grip strength meter. As a result, endurance EXE training reinforced muscular strength (both grip strength and grip strength/body weight), and regular aerobic EXE performance during DOX chemotherapy prevented a more severe reduction in the DOX-induced decrease in muscular strength. The grip strength test is more convenient and provides less stress to animals than other tests, including in vivo measurement of muscle force, the wire hang test, and the vertical pole test [40]. However, the grip strength test uses the forelimb grip strength as an indicator of muscle function [41-43]. This study also presented forelimb grip strength values, but the targeted GAS muscle for the biological actions was involved in the hindlimb compartments. Although recent evidence has demonstrated a positive correlation between grip strength and global muscle strength in older adults, little is known about this relationship in animals. Thus, further studies are required to explore whether a significant difference exists between forelimb/hindlimb grip strength and targeted gene expression profiles in the same or different muscle groups.
As mentioned above, verifying which muscles are susceptible to adaptations to specific interventions such as drugs and exercise is a prerequisite. This experimental platform demonstrated that the red portion of the GAS muscle tissues exhibited a proper adaptive response through increased CS activity. In contrast, although GAS muscle tissues were used similarly to those used in this study, there were no changes in CS activity [14]. In this regard, a significant difference might have been obtained if the red GAS muscles were used in this study and whether the EXE training protocol was sufficient to increase aerobic capacity. Thus, exercise scientists must be aware of exercise-induced adaptational effects.
Next, this study examined whether endurance EXE training prevented DOX-induced autophagy dysfunction under skeletal muscle wasting conditions. Indeed, the physiological regulation of endurance EXE-induced autophagy signaling pathways in skeletal muscle is well-described [44,45], but pathological modulations parallel to these themes are needed to investigate the overall autophagy flux with prominent biomarkers. Thus, this study examined the induction of autophagy (BECN1), autophagosome formation (ATG7, LC3B I to II turnover, and p62), and autophagosome fusion with lysosomes (LAMP2) for lysosomal degradation (CTSL). In this regard, chronic DOX chemotherapy inhibits autophagy induction and thereby contributes to the pathogenesis of muscle wasting, but EXE-training adaptations recover by enhancing basal autophagy and lysosomal function with the upregulation of LC3B-II and LAMP2, respectively. LC3BII is a key autophagy marker; however, it is also strongly associated with mitophagy, the selective autophagy of damaged mitochondria that maintains mitochondria homeostasis [46]. These data show a significant increase in LC3B-II protein expression in the DOX-EXE group. This appears to be a summation effect of the dual functions of LC3. Indeed, endurance exercise training-induced adaptations increase âautophagyâ as well as âmitophagyâ in skeletal muscles [47,48]. Attempting to ascertain the relevance of endurance training-induced mitophagy in DOX-induced muscle wasting would have clearly explained this snapshot. However, there are indirect supporting data showing a higher content of mitochondrial oxidative phosphorylation (OXPHOS) in response to 4 weeks of endurance exercise after chronic DOX-receiving in mice [20]. DOX directly affects mitochondrial superoxide production, causing myotoxicity and muscle wasting [49,50]. Therefore, they may be strongly associated with mitophagy. LAMP2 is involved in the partitioning of macroautophagy, but is also involved in chaperone-mediated autophagy (CMA) [51]. Thus, these results may indicate a compensatory mechanism involving the functional redundancy of LC3B-II and LAMP2.
Contrary to expectations, this study was not sufficient to monitor auto(phago)lysosomes with no changes in CTSL among groups, while an initial study showed significantly decreased CTSL content in pathogenic muscle-wasting mice [52,53] and Glucocorticoid-induced muscle atrophy and disuse atrophy of skeletal muscles were observed by increased transcriptional levels of cathepsin B and D [54,55]. Moreover, Smuder AJ and his colleagues examined cathepsin B, D, and L mRNA and CTSL protein levels in male SD rats with a single bout of DOX treatment and preconditioning EXE, but their data showed only significant changes in CTSL after interventions [29]. Because of these inconsistent results, the author should consider some of the major lysosomal proteases, not only CTSL, but also other cathepsins B, D, and H, to determine the proteolytic capacity of lysosomes [56]. Thus, it is necessary to examine at least three types of cathepsins B, D, and L, to demonstrate their biochemical functions under pathological conditions.
To potentially operate the muscle repair/regeneration machinery under skeletal muscle wasting conditions, it is crucial to activating the SC and functional ability of the MRF. This study found that the sequence of SC activation and myogenesis was not significantly altered in either the DOX-receiving or EXE-training groups, but aerobic EXE adaptations enhanced MYOD expression. It seems to be a self-renewal mechanism by a non-canonical pathway, such as Wnt7a [57], a promising candidate gene against cancer cachexia [58], which might be a plausible explanation for promoting the differentiation of neighboring cells by secreting MYOD out of myocytes, because Wnt3a can directly regulate MYOD expression [59]. Additionally, further experiments must verify the translocational changes between the cytosol and nucleus to better understand the functional redundancy of MRF, as MYOD and MYOG were discovered in myonuclei located in the euchromatin, myofibrils, and nucleus [60].
Finally, it is necessary to confirm whether the most common metabolic determinants are relevant to controlling EXE training adaptations for DOX-induced muscle wasting. These metabolic signaling pathways have been explored as crucial regulators of protein synthesis and degradation, which can lead to changes in muscle mass and function [22-24,61]. A previous study suggested that acute (2 weeks) and moderate intensity (60% maximal velocity) of EXE performance until the animalâs exhaustion escalated AMPK activation and contributed to eliminating DOX-induced harmful effects [14], while this study showed chronic (8 weeks) and high intensity (VO2max 70-80%) EXE treadmill running with an increase in CS protein levels was not associated with the upregulation of AMPK activity. These discrepancies are probably associated with different exercise intensities, but not duration, as the most recent meta-analysis evaluated muscle metabolites and exercise intensity as the most important predictors of AMPK activation [62].
Another study demonstrated that chronic DOX restrained mTOR activity and reduced the CSA in MHC-I and MHCII myofibers, but 6 weeks of endurance training protected against these side effects [15]. However, this study revealed that neither DOX nor EXE was associated with the AKT/mTOR signaling pathway, even though AKT induced alterations in both total AKT and p-AKTSer473, but the ratio of p-AKTSer473/AKT was blunted. For these discrepancies compared with previous studies, there were different gradients of interventions: an accumulative volume of DOX (i.e., other studies: 7.5 mg/kg or 12 mg/kg vs. this study: 25 mg/kg) and the EXE training protocol (i.e., frequency, intensity, time, and duration). Although the candidate metabolic determinants of EXE training adaptation effects during DOX-induced muscle wasting are not clearly understood, there is a hopeful finding that the combination of EXE training and nutritional supplementation such as resveratrol (to ameliorate DOX-induced toxicity and to boost EXE performance as an antioxidant) may contribute to more beneficial effects on metabolic function in cardiac injury in mice [63].
If this study used tumor-bearing mice to mimic the pathophysiological conditions of patients with cancer, it would be the best experimental in vivo study. Although the current study used non-tumor-bearing mice as a limitation, the author suggests that this is a meaningful trial because it proved acceptable for long-term endurance exercise training while undergoing chronic chemotherapy with some beneficial skeletal muscle metabolism. Moreover, an impactful and reliable preclinical study using genetically invented breast cancer mouse models indicated that exercise training prevented DOX-induced tumor growth [64]. Thus, further studies using tumor-bearing mice are required to provide more valuable data.
Taken together, this study confirms that chronic DOX chemotherapy induces muscle wasting, but long-term aerobic EXE training enhances muscular strength by improving the functional redundancy of autophagy and repairing myogenic differentiation. Therefore, moderate-intensity aerobic EXE training could be recommended for patients receiving cancer chemotherapy to improve muscle quality, which leads to the maintenance of activities of daily living and contributes to the long-term survival rate of patients with cancer.
ACKNOWLEDGMENTS
This work was supported by the Ministry of Education of the Republic of Korea and the National Research Foundation of Korea (NRF-2018S1A5B5A02037951). I declare no conflicts of interest regarding the publication of this article. In addition, I thank Prof. Hyung Joo Suh (School of Biosystems and Biomedical Sciences, Korea University, Republic of Korea) for providing me with the grip strength meter. Lastly, I would like to thank Editage (www.editage.co.kr) for English language editing.
Figure 1.
Skeletal muscle strength test in mice. (A) A representative image for the grip strength test, (B) quantification of grip strength in each group, and (C) The ratio of grip strength/body weight in each group. All mice were assigned seven per group. SAL, saline; DOX, doxorubicin; SED, sedentary; EXE, exercise training. Significant differences are denoted by an asterisk for p < 0.01(**) and p < 0.001(***). Data are presented as mean Âą SEM.
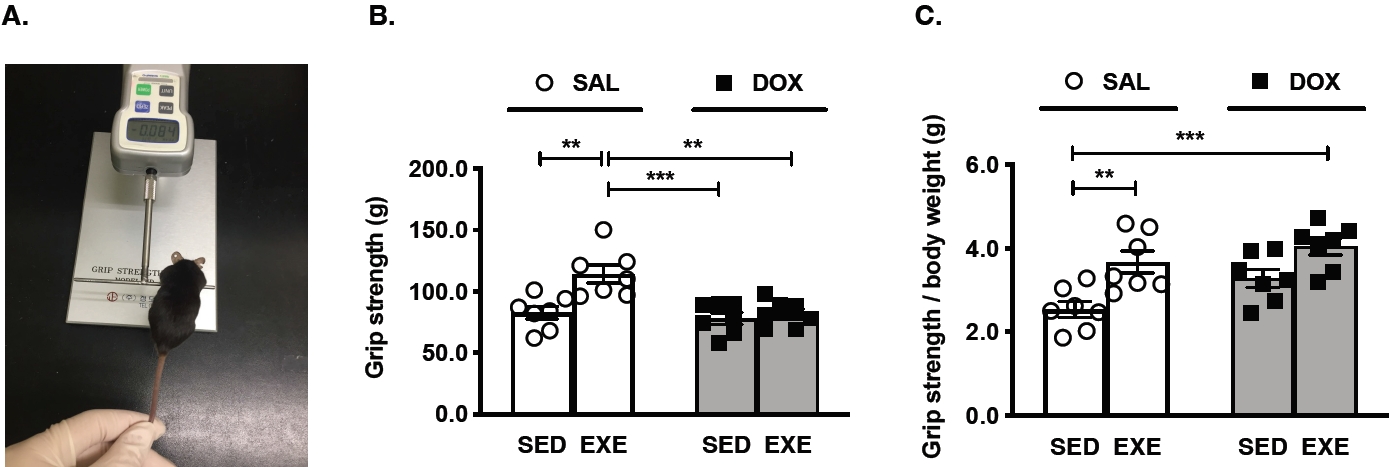
Figure 2.
Effects of endurance exercise training-induced adaptations during DOX chemotherapy on the key regulators of autophagy in skeletal muscle. (A) Representative western blot images are shown on the left side of the graph. Quantifications of (B) CS, (C) BECN1, (D) ATG 7, (E) p62, (F) LC3B-II, LC3B-I, and LC3B II/I ratio, (G) LAMP2, and (H) CTSL protein levels. All target proteins were normalized by Ponceau-stained total proteins (n=6-7 mice per group). SAL, saline; DOX, doxorubicin; SED, sedentary; EXE, exercise training. CS, Citrate synthase; BECN1, Beclin-1; LAMP2, Lysosome-associated membrane protein 2; CTSL, Cathepsin L.
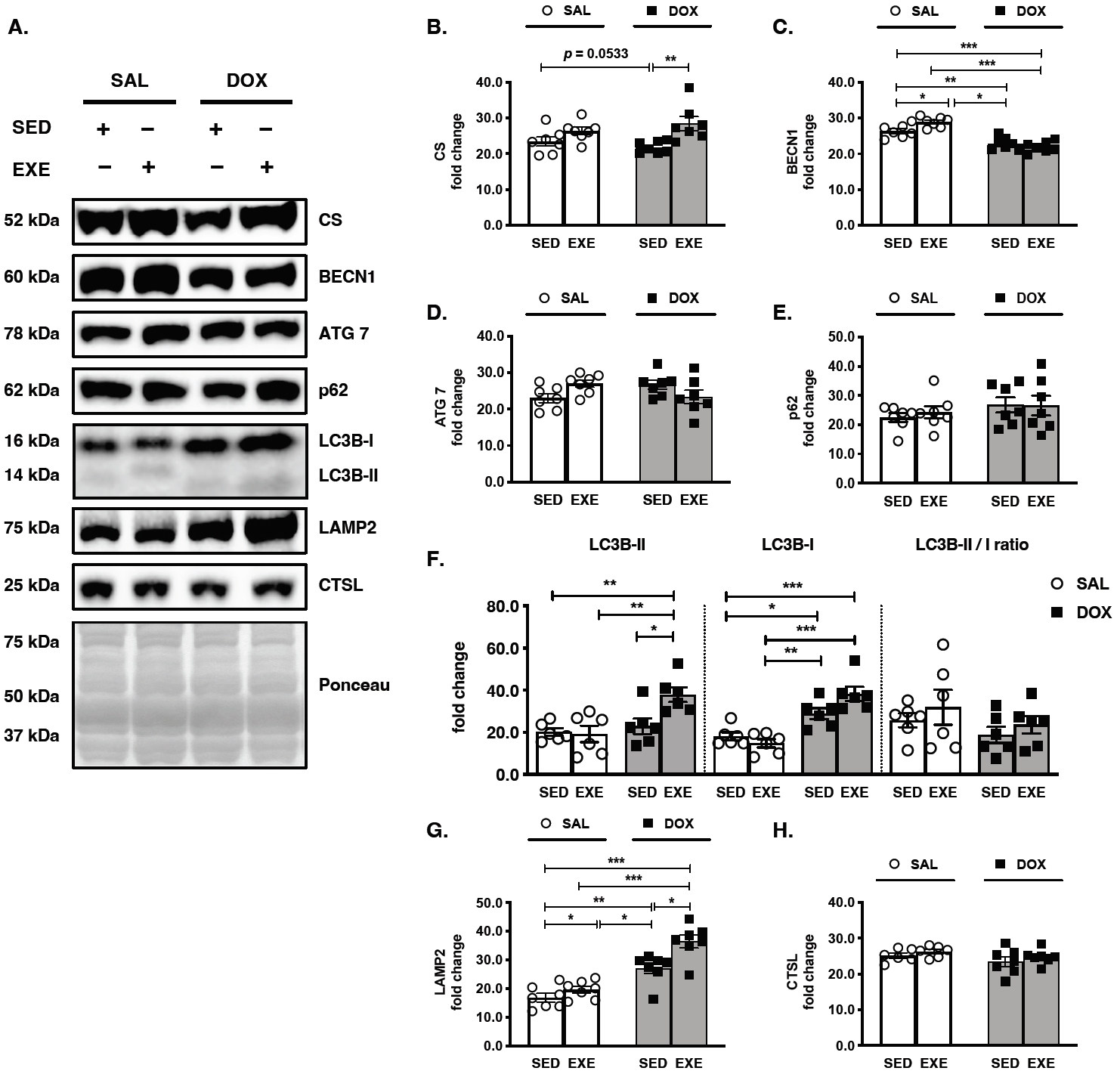
Figure 3.
Effects of endurance exercise training-induced adaptations during DOX chemotherapy on myogenic regulatory factors (MRF) and antioxidant enzymes in skeletal muscle. (A) Representative western blot images are shown on the left side of the graph. Quantifications of (B) PAX7, MYOD, and MYOG, (C) SOD1, and (D) SOD2 protein levels. All target proteins were normalized by Ponceau-stained total proteins (n=6-7 mice per group). SAL, saline; DOX, doxorubicin; SED, sedentary; EXE, exercise training. PAX 7, paired box protein 7; MYOD, myogenic differentiation 1; MYOG, myogenin; SOD1, superoxide dismutase 1; SOD2, superoxide dismutase 2.
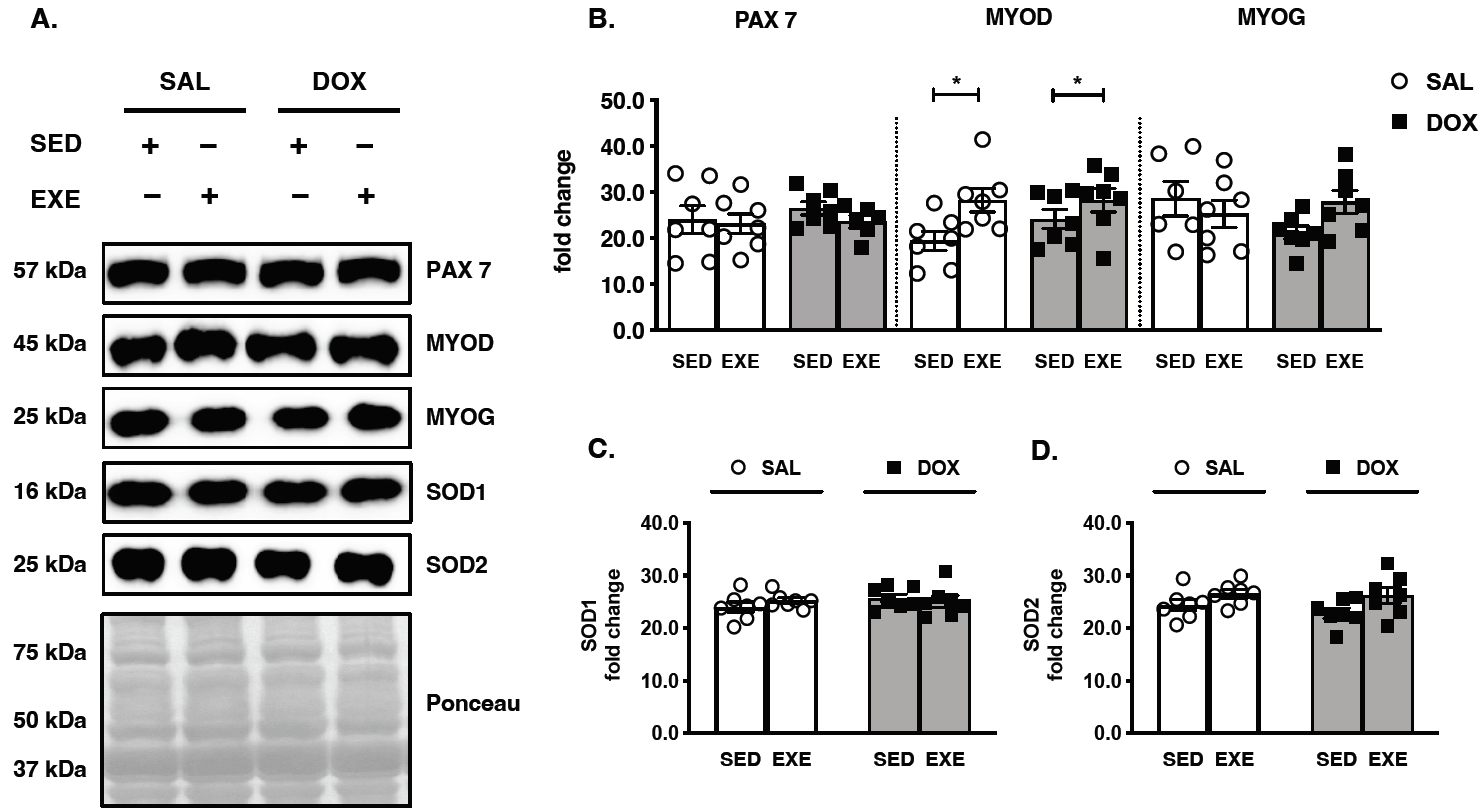
Figure 4.
Effects of endurance exercise training-induced adaptations during DOX chemotherapy on metabolic determinants in skeletal muscle (A) Representative western blot images are shown on the left side of the graph. Quantifications of (B) p-AMPKThr172, AMPK, and p-AMPKThr172/AMPK ratio, (C) p-AKTThr308, AKT, and p-AKTThr308/AKT ratio, (D) p-AKTSer473, AKT, and p-AKTSer473/AKT ratio, (E) p-mTORSer2448, mTOR, and p-mTORSer2448/ mTOR ratio. All target proteins were normalized by Ponceau-stained total proteins (n=6-7 mice per group). SAL, saline; DOX, doxorubicin; SED, sedentary; EXE, exercise training. AMP-activated protein kinase, AMPK; Mammalian target of rapamycin, mTOR.
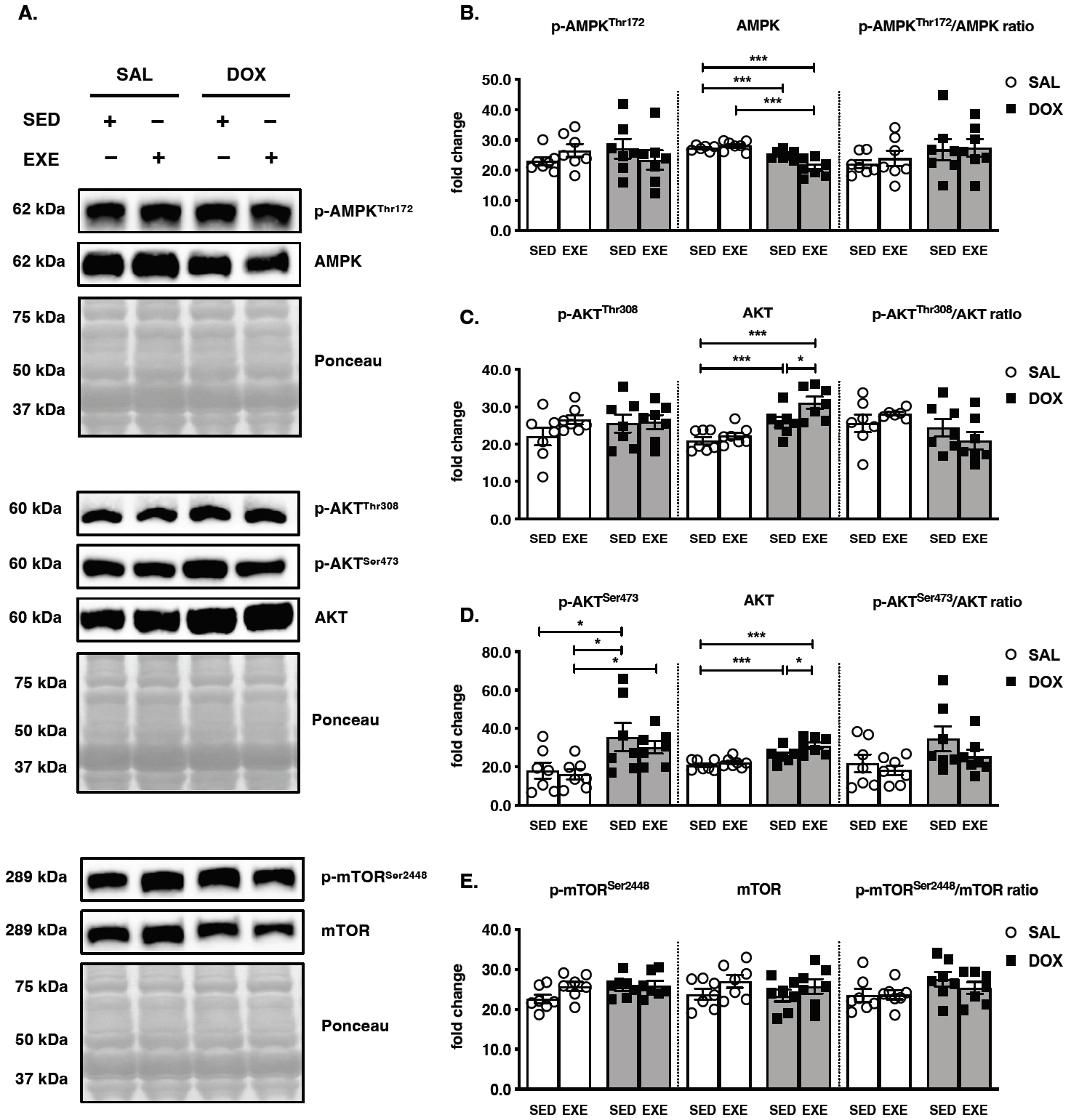
Table 1.
Changes in body weight and skeletal muscle weight for each group.
SED-SAL, sedentary group treated with saline; EXE-SAL, exercise training group treated with saline; SED-DOX, sedentary group treated with doxorubicin; EXE-DOX, exercise training group treated with doxorubicin (n=7 animals per each group). B.W, body weight; GAS, gastrocnemius; M.W, muscle weight; Data are presented as means Âą SEM. Different superscripts (a and b) represent significant differences at p<0.05.
REFERENCES
1. ArgilĂŠs JM, Busquets S, Stemmler B, LĂłpez-Soriano FJ. Cancer cachexia: understanding the molecular basis. Nat Rev Cancer 2014;14:754-62.



2. Yeom E, Yu K. Understanding the molecular basis of anorexia and tissue wasting in cancer cachexia. Exp Mol Med 2022;54:426-32.




3. Ni J, Zhang L. Cancer cachexia: definition, staging, and emerging treatments. Cancer Manag Res 2020;12:5597-605.


4. Cheong IY, Yoo JS, Chung SH, Park SY, Song HJ, Lee JW, Hwang JH. Functional loss in daily activity in ovarian cancer patients undergoing chemotherapy. Arch Gynecol Obstet 2019;299:1063-9.



5. Wang S, Konorev EA, Kotamraju S, Joseph J, Kalivendi S, Kalyanaraman B. Doxorubicin induces apoptosis in normal and tumor cells via distinctly different mechanisms. intermediacy of H(2)O(2)- and p53-dependent pathways. J Biol Chem 2004;279:25535-43.

6. Zhang S, Liu X, Bawa-Khalfe T, Lu LS, Lyu YL, Liu LF, Yeh ET. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat Med 2012;18:1639-42.



7. Meredith AM, Dass CR. Increasing role of the cancer chemotherapeutic doxorubicin in cellular metabolism. J Pharm Pharmacol 2016;68:729-41.



8. Hayward R, Hydock D, Gibson N, Greufe S, Bredahl E, Parry T. Tissue retention of doxorubicin and its effects on cardiac, smooth, and skeletal muscle function. J Physiol Biochem 2013;69:177-87.



9. Min K, Kwon OS, Smuder AJ, Wiggs MP, Sollanek KJ, Christou DD, Yoo JK, Hwang MH, Szeto HH, Kavazis AN, Powers SK. Increased mitochondrial emission of reactive oxygen species and calpain activation are required for doxorubicin-induced cardiac and skeletal muscle myopathy. J Physiol 2015;593:2017-36.




10. Hiensch AE, Bolam KA, Mijwel S, Jeneson JAL, Huitema ADR, Kranenburg O, van der Wall E, Rundqvist H, Wengstrom Y, May AM. Doxorubicin-induced skeletal muscle atrophy: elucidating the underlying molecular pathways. Acta Physiol (Oxf) 2020;229:e13400.



11. DâLugos AC, Fry CS, Ormsby JC, Sweeney KR, Brightwell CR, Hale TM, Gonzales RJ, Angadi SS, Carroll CC, Dickinson JM. Chronic doxorubicin administration impacts satellite cell and capillary abundance in a muscle-specific manner. Physiol Rep 2019;7:e14052.




12. Gilliam LA, Lark DS, Reese LR, Torres MJ, Ryan TE, Lin CT, Cathey BL, Neufer PD. Targeted overexpression of mitochondrial catalase protects against cancer chemotherapy-induced skeletal muscle dysfunction. Am J Physiol Endocrinol Metab 2016;311:E293-301.



13. McGrath MJ, Eramo MJ, Gurung R, Sriratana A, Gehrig SM, Lynch GS, Lourdes SR, Koentgen F, Feeney SJ, Lazarou M, McLean CA, Mitchell CA. Defective lysosome reformation during autophagy causes skeletal muscle disease. J Clin Invest 2021;131:e135124.



14. de Lima EA, de Sousa LGO, de STAA, Marshall AG, Zanchi NE, Neto JCR. Aerobic exercise, but not metformin, prevents reduction of muscular performance by AMPk activation in mice on doxorubicin chemotherapy. J Cell Physiol 2018;233:9652-62.



15. Dickinson JM, DâLugos AC, Mahmood TN, Ormsby JC, Salvo L, Dedmon WL, Patel SH, Katsma MS, Mookadam F, Gonzales RJ, Hale TM, Carroll CC, Angadi SS. Exercise protects skeletal muscle during chronic doxorubicin administration. Med Sci Sports Exerc 2017;49:2394-403.


17. Schmidt M, Schuler SC, Huttner SS, von Eyss B, von Maltzahn J. Adult stem cells at work: regenerating skeletal muscle. Cell Mol Life Sci 2019;76:2559-70.




18. HernĂĄndez-HernĂĄndez JM, GarcĂa-GonzĂĄlez EG, Brun CE, Rudnicki MA. The myogenic regulatory factors, determinants of muscle development, cell identity and regeneration. Semin Cell Dev Biol 2017;72:10-8.



19. Quinn CJ, Hydock DS. Effects of endurance exercise and doxorubicin on skeletal muscle myogenic regulatory factor expression. Muscles Ligaments Tendons J 2018;7:418-25.



20. Kwon I. Protective effects of endurance exercise on skeletal muscle remodeling against doxorubicin-induced myotoxicity in mice. Phys Act Nutr 2020;24:11-21.



21. Lantier L, Fentz J, Mounier R, Leclerc J, Treebak JT, Pehmøller C, Sanz N, Sakakibara I, Saint-Amand E, Rimbaud S, Maire P, Marette A, Ventura-Clapier R, Ferry A, Wojtaszewski JF, Foretz M, Viollet B. AMPK controls exercise endurance, mitochondrial oxidative capacity, and skeletal muscle integrity. FASEB J 2014;28:3211-24.



22. Kjøbsted R, Hingst JR, Fentz J, Foretz M, Sanz MN, Pehmøller C, Shum M, Marette A, Mounier R, Treebak JT, Wojtaszewski JFP, Viollet B, Lantier L. AMPK in skeletal muscle function and metabolism. FASEB J 2018;32:1741-77.




23. Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, Zlotchenko E, Scrimgeour A, Lawrence JC, Glass DJ, Yancopoulos GD. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol 2001;3:1014-9.



24. Jaiswal N, Gavin MG, Quinn WJ, Luongo TS, Gelfer RG, Baur JA, Titchenell PM. The role of skeletal muscle Akt in the regulation of muscle mass and glucose homeostasis. Mol Metab 2019;28:1-13.



25. Wei Y, Zhou J, Yu H, Jin X. AKT phosphorylation sites of Ser473 and Thr308 regulate AKT degradation. Biosci Biotechnol Biochem 2019;83:429-35.



26. Kang SH, Lee HA, Kim M, Lee E, Sohn UD, Kim I. Forkhead box O3 plays a role in skeletal muscle atrophy through expression of E3 ubiquitin ligases MuRF-1 and atrogin-1 in Cushingâs syndrome. Am J Physiol Endocrine Metab 2017;312:E495-e507.

27. Kwon I, Go GW, Lee Y, Kim JH. Prolonged endurance exercise adaptations counteract doxorubicin chemotherapy-induced myotoxicity in mice. Appl Sci 2022;12:3652.

28. Kavazis AN, Smuder AJ, Powers SK. Effects of short-term endurance exercise training on acute doxorubicin-induced FoxO transcription in cardiac and skeletal muscle. J Appl Physiol (1985) 2014;117:223-30.



29. Smuder AJ, Kavazis AN, Min K, Powers SK. Exercise protects against doxorubicin-induced markers of autophagy signaling in skeletal muscle. J Appl Physiol (1985) 2011;111:1190-8.


30. Smuder AJ, Kavazis AN, Min K, Powers SK. Exercise protects against doxorubicin-induced oxidative stress and proteolysis in skeletal muscle. J Appl Physiol (1985) 2011;110:935-42.



31. Mackay AD, Marchant ED, Munk DJ, Watt RK, Hansen JM, Thomson DM, Hancock CR. Multitissue analysis of exercise and metformin on doxorubicin-induced iron dysregulation. Am J Physiol Endocrinol Metab 2019;316:E922-30.


32. Huertas AM, Morton AB, Hinkey JM, Ichinoseki-Sekine N, Smuder AJ. Modification of neuromuscular junction protein expression by exercise and doxorubicin. Med Sci Sports Exerc 2020;52:1477-84.



33. Schefer V, Talan MI. Oxygen consumption in adult and AGED C57BL/6J mice during acute treadmill exercise of different intensity. Exp Gerontol 1996;31:387-92.


34. Ertunc M, Sara Y, Korkusuz P, Onur R. Differential contractile impairment of fast- and slow-twitch skeletal muscles in a rat model of doxorubicin-induced congestive heart failure. Pharmacology 2009;84:240-8.



35. Hydock DS, Lien CY, Jensen BT, Schneider CM, Hayward R. Characterization of the effect of in vivo doxorubicin treatment on skeletal muscle function in the rat. Anticancer Res 2011;31:2023-8.

36. Bredahl EC, Pfannenstiel KB, Quinn CJ, Hayward R, Hydock DS. Effects of Exercise on Doxorubicin-Induced Skeletal Muscle Dysfunction. Med Sci Sports Exerc 2016;48:1468-73.


37. Huang SC, Wu JF, Saovieng S, Chien WH, Hsu MF, Li XF, Lee SD, Huang CY, Huang CY, Kuo CH. Doxorubicin inhibits muscle inflammation after eccentric exercise. J Cachexia Sarcopenia Muscle 2017;8:277-84.



38. Egan B, Zierath JR. Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metab 2013;17:162-84.


39. Teixeira AAS, Biondo LA, Diniz TA, Silveira LS, Coletti D, Busquets Rius S, Rosa Neto JC. Exercise reduces the resumption of tumor growth and proteolytic pathwyas in the skeletal muscle of mice following chemotherapy. Cancers (Basel) 2020;12:3466.


40. Takeshita H, Yamamoto K, Nozato S, Inagaki T, Tsuchimochi H, Shirai M, Yamamoto R, Imaizumi Y, Hongyo K, Yokoyama S, Takeda M, Oguro R, Takami Y, Itoh N, Takeya Y, Sugimoto K, Fukada SI, Rakugi H. Modified forelimb grip strength test detects aging-associated physiological decline in skeletal muscle function in male mice. Sci Rep 2017;7:42323.




41. Girgis CM, Cha KM, Houweling PJ, Rao R, Mokbel N, Lin M, Clifton-Bligh RJ, Gunton JE. Vitamin D receptor ablation and vitamin D deficiency result in reduced grip strength, altered muscle fibers, and increased myostatin in mice. Calcif Tissue Int 2015;97:602-10.



42. Hsu YJ, Huang WC, Chiu CC, Liu YL, Chiu WC, Chiu CH, Chiu YS, Huang CC. Capsaicin supplementation reduces physical fatigue and improves exercise performance in mice. Nutrients 2016;8:648.



43. Wei L, Wen YT, Lee MC, Ho HM, Huang CC, Hsu YJ. Effects of isolated soy protein and strength exercise training on exercise performance and biochemical profile in postpartum mice. Metabolism 2019;94:18-27.


44. Rocchi A, He C. Regulation of exercise-induced autophagy in skeletal muscle. Curr Pathobiol Rep 2017;5:177-86.




45. Martin-Rincon M, Morales-Alamo D, Calbet JAL. Exercise-mediated modulation of autophagy in skeletal muscle. Scand J Med Sci Sports 2018;28:772-81.



46. Leduc-Gaudet JP, Hussain SNA, Barreiro E, Gouspillou G. Mitochondrial dynamics and mitophagy in skeletal muscle health and aging. Int J Mol Sci 2021;22:8179.



47. Sanchez AM, Bernardi H, Py G, Candau RB. Autophagy is essential to support skeletal muscle plasticity in response to endurance exercise. Am J Physiol Regul Integr Comp Physiol 2014;307:R956-69.


48. Carter HN, Kim Y, Erlich AT, Zarrin-Khat D, Hood DA. Autophagy and mitophagy flux in young and aged skeletal muscle following chronic contractile activity. J Physiol 2018;596:3567-84.




49. Rybalka E, Timpani CA, Cheregi BD, Sorensen JC, Nurgali K, Hayes A. Chemotherapeutic agents induce mitochondrial superoxide production and toxicity but do not alter respiration in skeletal muscle in vitro. Mitochondrion 2018;42:33-49.


50. Sorensen JC, Cheregi BD, Timpani CA, Nurgali K, Hayes A, Rybalka E. Mitochondria: inadvertent targets in chemotherapy-induced skeletal muscle toxicity and wasting? Cancer Chemother Pharmacol 2016;78:673-83.



51. Losmanovå T, Janser FA, Humbert M, Tokarchuk I, Schläfli AM, Neppl C, Schmid RA, Tschan MP, Langer R, Berezowska S. Chaperone-mediated autophagy markers LAMP2A and HSC70 are independent adverse prognostic markers in primary resected squamous cell carcinomas of the lung. Oxid Med Cell Longev 2020;2020:8506572.


52. Deval C, Mordier S, Obled C, Bechet D, Combaret L, Attaix D, Ferrara M. Identification of cathepsin L as a differentially expressed message associated with skeletal muscle wasting. Biochem J 2001;360:143-50.




53. Combaret L, BĂŠchet D, Claustre A, Taillandier D, Richard I, Attaix D. Down-regulation of genes in the lysosomal and ubiquitin-proteasome proteolytic pathways in calpain-3-deficient muscle. Int J Biochem Cell Biol 2003;35:676-84.


54. Dardevet D, Sornet C, Taillandier D, Savary I, Attaix D, Grizard J. Sensitivity and protein turnover response to glucocorticoids are different in skeletal muscle from adult and old rats. Lack of regulation of the ubiquitin-proteasome proteolytic pathway in aging. J Clin Invest 1995;96:2113-9.



55. Taillandier D, Aurousseau E, Meynial-Denis D, Bechet D, Ferrara M, Cottin P, Ducastaing A, Bigard X, Guezennec CY, Schmid HP. Coordinate activation of lysosomal, Ca 2+-activated and ATP-ubiquitin-dependent proteinases in the unweighted rat soleus muscle. Biochem J 1996;316:65-72.




56. Bechet D, Tassa A, Taillandier D, Combaret L, Attaix D. Lysosomal proteolysis in skeletal muscle. Int J Biochem Cell Biol 2005;37:2098-114.


57. von Maltzahn J, Chang NC, Bentzinger CF, Rudnicki MA. Wnt signaling in myogenesis. Trends Cell Biol 2012;22:602-9.



58. Schmidt M, Poser C, von Maltzahn J. Wnt7a counteracts cancer cachexia. Mol Ther Oncolytics 2020;16:134-46.



59. Pan YC, Wang XW, Teng HF, Wu YJ, Chang HC, Chen SL. Wnt3a signal pathways activate MyoD expression by targeting cis-elements inside and outside its distal enhancer. Biosci Rep 2015;35:e00180.




60. Ishido M, Kami K, Masuhara M. Localization of MyoD, myogenin and cell cycle regulatory factors in hypertrophying rat skeletal muscles. Acta Physiol Scand 2004;180:281-9.


61. Thomson DM. The role of AMPK in the regulation of skeletal muscle size, hypertrophy, and regeneration. Int J Mol Sci 2018;19:3125.



62. Rothschild JA, Islam H, Bishop DJ, Kilding AE, Stewart T, Plews DJ. Factors influencing AMPK activation during cycling exercise: a pooled analysis and meta-regression. Sports Med 2022;52:1273-94.



-
METRICS

-
- 2 Crossref
- Scopus
- 1,701 View
- 41 Download
- Related articles in Phys Act Nutr





