INTRODUCTION
Recently, the sports-related industry has experienced growth as more people enjoy leisure and sports activities. Sports mania involves the demand for more specialized knowledge, rather than participating in sports as a hobby. Ordinary people, as well as professional athletes, are increasingly becoming interested in developing sports abilities, which has resulted in increased demand for erogogenic aids. The consumption of protein supplements, such as whey protein concentrates, egg white Albumen, BCAA (Branched chain Amino Acids), glutamine, and creatine have gradually been increasing. Protein, or amino acid supplements, promote protein synthesis by activating hormones and signaling molecules [1,2], while the synthesis of protein enables recovery from exercise induced damage and improves exercise performance by increasing the muscle volume [3].
Resistance exercise and intake of appropriate nutrition for muscle protein synthesis are important factors, as resistance exercise leads to the synthesis of protein in the muscles in 24-48 hours [4-6]. Regardless of age and sex, resistance exercise and appropriate intake of nutrients were found to increase muscle protein synthesis [2,8]. Synthesis is controlled by intracellular signaling pathways. If the protein synthetic signaling response is active at a higher rate than degradation, muscle is built.
The mTOR is known as an important signaling molecule involving muscle hypertrophy [9]. Exercise and consumption of amino acids, especially leucine, function directly with mTOR, rather than through the pathways of growth factors such as IGF-1. During resistance exercise, mTOR activation is inhibited by AMPK (AMP-Activated protein kinase); thus, limited protein synthesis occurs as increasing amounts of amino acids are used for energy metabolism. During the recovery period, AMPK activation is decreased and mTOR reaches peak activity [10,11]. The mTOR exists as two types: mTORC1 (mTOR complex 1), which is sensitive to rapamycin, and insensitive mTORC2 (mTOR complex 2). The mTORC1 consists of mTOR, raptor, and G╬▓L (G-protein ╬▓-subunit-like protein), which control the translation of mRNA through phosphorylation of S6K1, eIF (eukaryotic initiation factor) 4E, and 4E-BP1 (4E binding protein 1). The mTORC1 can be activated by amino acids (especially leucine), hormones (IGF-1), and energy metabolic signals [12,13].
Amino acid metabolism in skeletal muscle is limited to glutamate, aspartate, asparagine, leucine, isoleucine, and valine. Among them, leucine is considered as the most remarkable amino acid related to the synthesis of muscle contractile protein [4,14].
Leucine regulates the PI3-Kinase (phosphoinositol 3-kinase) signal cascade by functioning as a source of nitrogen for alanine and glutamine to be used in muscle contractile protein synthesis. The initiation of translation, insulin signaling, and the possible contribution of leucine in the synthesis of alanine and glutamine are dependent on the intracellular concentrations. The intracellular concentration of leucine appears to be determined according to the balance among the intracellular protein degradation, absorption rate of leucine from plasma, intracellular protein synthesis and rate of leucine removal through amino acid oxidation [15].
The intracellular leucine concentration contributes to the control of energy metabolism through the BCKDH (branched-chain ╬▒-keto acid) pathway, while functioning as the control of insulin secretion via pyruvate dehydrogenase, IRS-1 (insulin receptor substrate-1), and ╬▓-cell stimulating path. Leucine is also involved in muscle protein synthesis through activation of mTOR, eIF4G, and eEF2 signaling proteins [16]. The mTOR signaling pathway can be activated by leucine to begin protein synthesis in the absence of insulin activation [17,18]. Therefore, the interest in leucine among BCAA is increasing as a sports supplement. Leucine, Isoleucine, and Valine have been introduced at increased leucine ratios of 4:1:1, 8:1:1 and even 12:1:1, compared to the previous ratio of 2:1:1.
The amount of leucine for supplementation was varied in previous studies, with Anthony et al. [1,19], Crozier et al. [20], and Layman [21] applying leucine uptakes in the range of 0.068~1.35 g/kg for dietary restricted (food deprived, fasting) rats, or 1 mmol/kg, which is the physiological concentration of leucine [22-24]. However, the appropriate intake volume for increasing muscle mass has not yet been determined. The present study evaluated the appropriate volume for leucine intake to induce increased muscle mass, or muscle hypertrophy, and investigated a possible synergic effect of leucine intake during exercise on muscle protein synthesis. Therefore, the present study aimed to clarify the effect of leucine on skeletal muscle mass following 8 weeks of resistance exercise using a climbing ladder [25]. The mass was measured by excision of the FHL, and the levels of PKB/Akt, mTOR, p70S6K, and 4EBP1 were analyzed.
METHODS
Animals
Forty-two nine-week-old male Sprague-Dawley rats (Samtako, Gyeonggi-do, South Korea) were used, The rats were divided into six groups, including the control group (CON; n = 7), 10% (L1; n = 7) and 50% (L5; n = 7) leucine administration groups, exercise training group (Ex; n = 7), exercise + 10% leucine administration group (EL1; n = 7), and exercise + 50% leucine administration group (EL5; n = 7). The experiment was started on the tenth week, after allowing one week for cage adaptation. The overall process of purchasing and breeding of animals was conducted with the approval of the Korea National Sport University Animal Experiment Ethics Committee (KNSU-IACUC-2012-03). Diet and fluid intake of the experimental animals were not restricted. All animals displaying disease, abnormal body mass, particularly excessive food intake, or lack of food intake were excluded from the experiment.
Exercise protocols
Resistance exercise training was performed using a climbing ladder (height 1m, inclination of 80 degrees), which was designed to make the rats ascend the ladder while imposing a constant load. Animals exercised on the ladder with free load for a week as a pre-training adaptation. Following adaptation, rats were loaded with 50% of 1RM (repetition maximum), equivalent to 50% of their initial body weight. The exercise was loaded as follows: one repetition of ladder exercise was conducted at 50%, 75%, 90%, and 100% of 1RM strength, after which 30g was added to each trial up to ten trials. Animals who could not continue climbing, or were hanging on the ladder, were considered to end the training before the tenth trial. The daily training intensity was determined based on the final load of the previous day as 1RM for the next day, and the exercise was started again with 50% of the final load of the previous day, applied in this manner up to the end of the experiment [25]. Resistance exercise was carried out three times a week for eight weeks.
Leucine administration
A water solution containing 54.0 g of L-leucine/L was prepared, and daily oral administration was done with 0.135 (10%) and 0.675 (50%) g/kg.wt. These dosages of leucine were set based on the amount of leucine ingested daily from food (1.35 g/kg.wt), which was set as 100%. In order to make amount of intake the same for the groups, an additional amount of saline was administrated to the 10% group. The control group was administrated with an equal amount of saline solution. Leucine was administered within 45 minutes after exercise, and the saline was also given at the same time using a Sonde.
Tissue sampling
All animals were sacrificed after anesthetized using an inhaled anesthetic (Isoplorane; AERANE) 48 hours after completion of the last exercise, which was 24 hours after the last administration of leucine and after 12 hours of fasting. After measuring the mass, the FHL was immediately excised, frozen in liquid nitrogen, and stored at -80Ōäā until analyzed.
Western blot analysis
Tissues were solubilized with a lysis buffer containing 1% SDS. Thirty g of protein from the lysates was then resolved by 12% SDS-PAGE, transferred to nitrocellulose membranes, incubated with specific antibodies and visualized by blotting with HRP-conjugated secondary antibodies.
Statistics
The mean and standard deviation was calculated using the statistical program PASW/PC+, Version 20.0. One-way analysis of variance (one-way ANOVA) was applied for comparison of the differences among groups, and two-way analysis of variance (Two-way ANOVA) was applied to identify interactions in accordance with the differences between exercise and leucine dose, after which post-hoc with LSD was applied. Statistical significance was set at p <.05.
RESULTS
Regarding body weight, no significant differences were observed among the groups; however, the body weight was increased by 33% after 8 weeks compared to the initial body weight. The greatest increase in body mass was observed in the group EL5 (138.67 g, 39.0%), while EL1 showed the least increase (92.29 g, 26%) among the groups (Fig. 1).
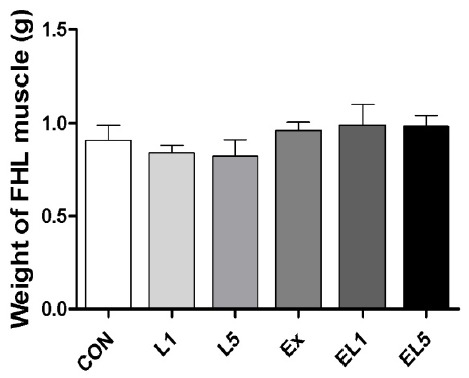
Analysis of the FHL mass also revealed no differences among the control group, leucine administration group, and exercise training group (Fig. 2); however, the relative FHL mass to body mass of the EX group was increased compared to the CON group and leucine administration groups (L1, L5) (p = .003), with the greatest difference occurring between the EL1 and CON group (pŌēż.001) (Fig. 3). While the EL5 group had lower relative FHL mas than the EL1 group, no significant differences were observed among the exercise groups.
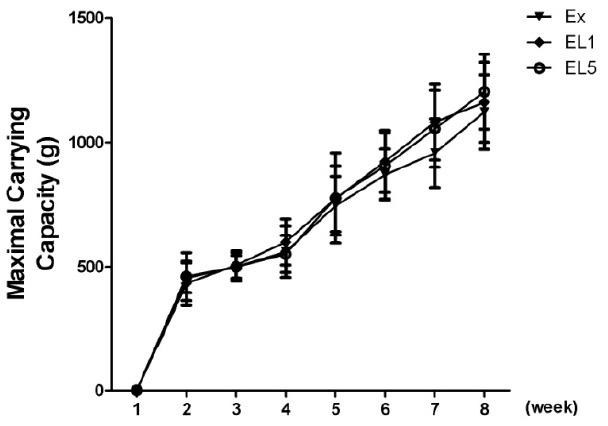
The maximum weekly exercise load of the exercise training group initially showed similar levels, as can be seen in Fig. 4. After eight weeks, a marked increase by 168% was observed in EL1 (434.1 ┬▒ 95.6 vs 1161.4 ┬▒ 149.8 g), while the EX group showed the least increase by 146% (456.5 ┬▒ 59.5 to 1122.6 ┬▒ 149.8 g). No significant difference was found between the EL1 and EL5 groups (168% vs 161%).
Next, the level of p-PKB/Akt was examined, as shown in Fig. 5. The leucine administration group displayed higher levels than the CON group. The L5 group showed significantly higher levels than the CON group (p = .002), while the difference between CON and L1 was not significant. For the combination of exercise and leucine administration, both the EL1 group (p = .034) and EL5 group (p = .002) displayed higher levels than the CON group. There was no interaction for Akt between exercise and the leucine administration (F(3) =.16, p = .854).
Analysis of p-mTOR (Ser2448) revealed higher levels in the leucine group than the CON group (Fig. 6); however, this difference was only significant for the L5 group (p = .004). In contrast, no difference was observed between the control CON group and the EX group, but for exercise combined with leucine administration, both EL1 (p = .001) and EL5 (p < .001) displayed higher levels than the CON group. The EL5 group also showed higher levels than the EX group (p = .003), but no difference was observed between EL1 and EL5. In addition, there was no interaction for p-mTOR (Ser2448) protein between exercise and the leucine administration (F(3) = .16, p = .854).
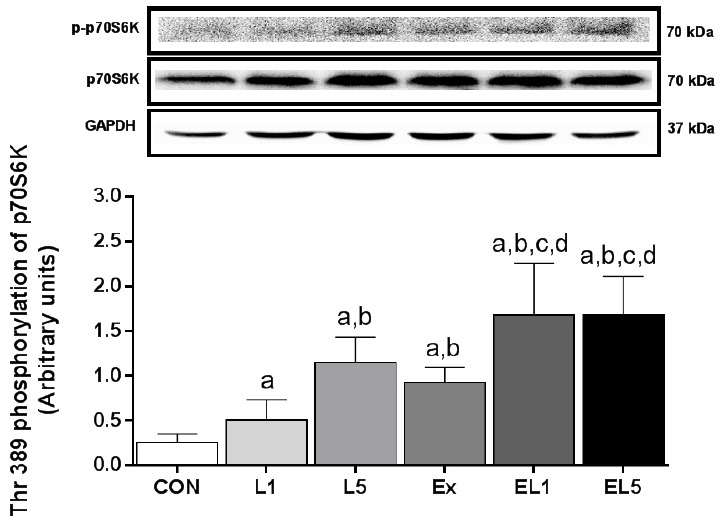
Similar to the other proteins examined, the EL5 group also exhibited higher levels of p70S6K protein in FHL muscle than the CON group (p < .001) (Fig. 7); however, the difference between EL5 and EL1 was also significant (p = .001). For the exercise groups, the EX group (p = .001), EL1 group (p < .001), and EL5 group (p < .001) all exhibited higher levels than the CON group. Further, the EL1 group (p < .001) and EL5 group (p < .001) were also higher than the EX group, while there was no difference between EL1 and EL5. When comparing the EX group with the leucine administration group, the EX group showed higher levels than L1 (p = .031), but no significant difference with L5 group. An interaction effect was found for p-p70S6K (Thr389) protein between exercise and leucine administration (F(3) = 3.440, p = .043).
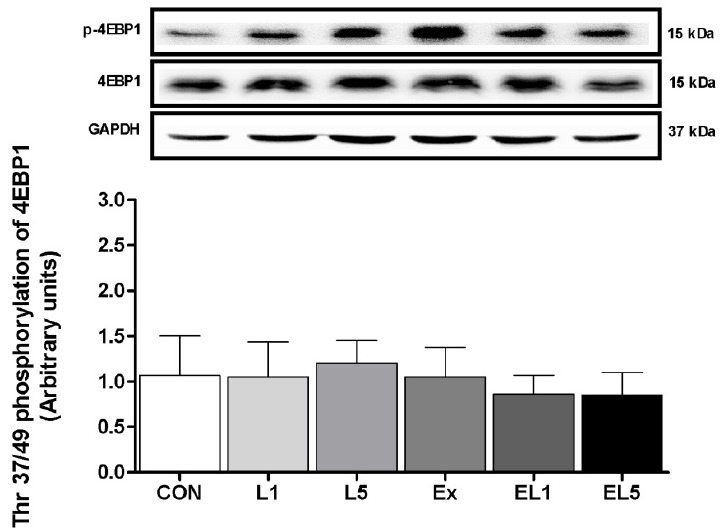
Unlike the other proteins examined, no differences were found among any of the groups regarding the expression of 4EBP1 protein in the FHL muscle, as shown in Fig. 8.
DISCUSSION
The major finding of the present study was that the relative body mass was increased in the EX groups compared with the CON group, while no significant differences were observed in muscle mass among the groups. Even though some signaling proteins were increased, which exhibited some differences among the groups, there were no significant differences in muscle mass between the leucine administration and exercise training combined with leucine administration groups in the present study. These findings were unexpected, in that leucine administration alone could not increase the muscle mass, and did not have additive effects for increase the muscle mass during exercise training. Our findings could not support the notion that leucine administration combined with exercise training may have an additive effect to increase muscle mass.
Nutrition intake is a crucial factor for muscle protein synthesis and hypertrophy, especially during resistance exercise [4]. Muscle protein synthesis replenishes the protein physiologically degraded, and allows muscle regeneration after structural damage caused during exercise, resulting in muscle hypertrophy [3]. For this reason, many athletes, as well as ordinary people interested in increasing muscle mass, take various nutritional supplements as ergogenic aids. Most nutritional supplements are taken in the form of protein and amino acid supplements.
However, even today, which nutrients at what dosage and duration of supplementation can allow an additive effect for increasing muscle mass still remain to be clarified. The present study was performed to suggest an appropriate dose of leucine for increasing muscle protein synthesis through analysis of PKB/Akt, mTOR, p70S6K, and 4EBP1 proteins.
In both the 10% leucine (L1) and 50% leucine (L5) administration groups, leucine dose-dependent increase in the expressions of PKB/Akt, mTOR, and p70S6K were observed compared to the control (CON) group. These results are supported by those of Crozier et al. [19], who observed increase in p70S6k activation even with a small amount (5%) of leucine administration in food-deprived SD rats, even though the protein synthesis was increased in a dose-dependent manner (10-100%), with significant increase at administration above 10%.
Gran et al. [28] reported that the mTOR signal in the leucine intake group was temporarily activated 30 minutes after intake, but this was not the case in the present study. The activation of PKB/Akt-mTOR-p70S6K was continued up to 24 hours in the present study.
The marked activation of PKB/Akt expression in the 10% leucine administration group (EL1) may not support the notion that leucine may activate muscle protein synthesis through the amino acid/leucine-mTORC1 signaling pathway, unlikely signaling expression through exercise, leading to the IRS1-PI3K-PDK- PKB/Akt-mTOR pathway. We speculate that the excessive administration of leucine (> 50%) may stimulates mTORC2, but this remains to be clarified.
Crozier et al. [19] also reported that more than administration of 10% leucine increased the level of 4EBP1 expression, while the activation of 4EBP1 was decreased after conducting a trial of resistance exercise [Dryer et al, 30]. The expression of 4EBP1 was reported to vary according to the protocol; however, this was not the case in the present study, as the levels of 4EBP1 remained unchanged in all groups. These disagreements may be accounted for due to the differences in time elapsed until measurement after administration, and/or the dosage employed. However, it should be further elucidated whether muscle protein synthesis occurs through 4EBP1 signaling during a certain duration after leucine administration, and definitively determined whether or not leucine administration leads to activation through the 4EBP1 pathway.
In the present study, the expressions of PKB/Akt, mTOR, and p70S6K were increased in both the EX group and the leucine administration group. These results support the observations of Hulmi et al. [2] that resistance exercise and endurance exercise activates the PKB/Akt-mTOR-p70S6K signaling pathways, while protein intake further enhances ths process.
However, the leucine administration strategy to help people who have difficulty performing physical activity or ageing people who have lowered physical activity may need to be reconsidered. Leucine administration itself may not be an alternative to prevent sarcopenia.
Herein, the leucine administration group did not show significant additive effects to the muscle mass. This is consistent with the results of the previous study by Hornberger et al. [25]. In particular, there were no differences between the EL5 and EL1 groups. It appeared that the relative body mass was increased in the EX group compared with the CON group (p = .003), while there was no significant difference in FHL mass among the groups. Even though some differences were observed in the signaling, no differences in muscle protein synthesis were observed between the leucine administration and exercise training combined with leucine administration groups in the present study.
CONCLUSION
The present study was designed to suggest the appropriate leucine intake to obtain the dual effects of restoring damaged muscle and enhancing muscle hypertrophy after muscular exercise training. It appeared that the relative body mass was increased in the EX group compared with the CON group, while no significant differences in muscle mass were found among the groups. This was an unexpected finding in that leucine administration alone could not increase the muscle mass, and did not have a possible additive effect to increase muscle mass during exercise training. In other words, our findings could not support the notion that leucine administration combined with exercise training may have an additive effect for increasing muscle mass. Even though some signaling proteins were increased, or some differences existed among groups, there were no differences in muscle mass between the leucine administration and exercise training combined with leucine administration groups in the present study. Future study may be needed to shed more light on this matter.