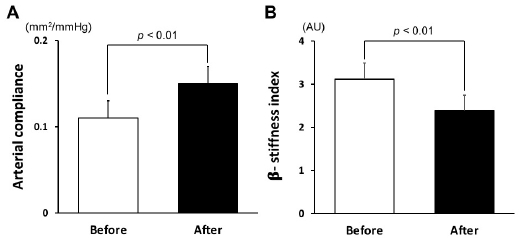
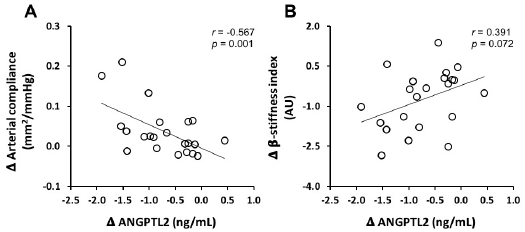
1.
Zebekakis PE, Nawrot T, Thijs L, Balkestein EJ, van der Heijden-Spek J, Van Bortel LM, Struijker-Boudier HA, Safar ME, Staessen JA. Obesity is associated with increased arterial stiffness from adolescence until old age.
J Hypertens. 2005;23:1839-46.

Zebekakis PE, Nawrot T, Thijs L, Balkestein EJ, van der Heijden-Spek J, Van Bortel LM, Struijker-Boudier HA, Safar ME, Staessen JA. Obesity is associated with increased arterial stiffness from adolescence until old age.
J Hypertens. 2005;23:1839-46. PMID:
10.1097/01.hjh.0000179511.93889.e9. PMID:
16148607.
2.
Safar ME, Czernichow S, Blacher J. Obesity, Arterial Stiffness, and Cardiovascular Risk.
J Am Soc Nephrol. 2006;17:S109-11.

Safar ME, Czernichow S, Blacher J. Obesity, Arterial Stiffness, and Cardiovascular Risk.
J Am Soc Nephrol. 2006;17:S109-11. PMID:
10.1681/asn.2005121321. PMID:
16565231.
3.
Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease.
Nat Rev Immunol. 2011;11:85-97.


Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease.
Nat Rev Immunol. 2011;11:85-97. PMID:
10.1038/nri2921. PMID:
21252989.
4.
Hotamisligil GS. Inflammation and metabolic disorders.
Nature. 2006;444:860-7.


Hotamisligil GS. Inflammation and metabolic disorders.
Nature. 2006;444:860-7. PMID:
10.1038/nature05485. PMID:
17167474.
5.
Miyaki A, Maeda S. Arterial stiffness and lifestyle modification.
J Phys Fit Sports Med. 2012;1:205-10.

Miyaki A, Maeda S. Arterial stiffness and lifestyle modification.
J Phys Fit Sports Med. 2012;1:205-10. PMID:
10.7600/jpfsm.1.205.
6.
Mahmud A, Feely J. Arterial stiffness is related to systemic inflammation in essential hypertension.
Hypertension. 2005;46:1118-22.

Mahmud A, Feely J. Arterial stiffness is related to systemic inflammation in essential hypertension.
Hypertension. 2005;46:1118-22. PMID:
10.1161/01.hyp.0000185463.27209.b0. PMID:
16216991.
7.
Tabata M, Kadomatsu T, Fukuhara S, Miyata K, Ito Y, Endo M, Urano T, Zhu HJ, Tsukano H, Tazume H, Kaikita K, Miyashita K, Iwawaki T, Shimabukuro M, Sakaguchi K, Ito T, Nakagata N, Yamada T, Katagiri H, Kasuga M, Ando Y, Ogawa H, Mochizuki N, Itoh H, Suda T, Oike Y. Angiopoietin-like protein 2 promotes chronic adipose tissue inflammation and obesity-related systemic insulin resistance.
Cell Metab. 2009;10:178-88.

Tabata M, Kadomatsu T, Fukuhara S, Miyata K, Ito Y, Endo M, Urano T, Zhu HJ, Tsukano H, Tazume H, Kaikita K, Miyashita K, Iwawaki T, Shimabukuro M, Sakaguchi K, Ito T, Nakagata N, Yamada T, Katagiri H, Kasuga M, Ando Y, Ogawa H, Mochizuki N, Itoh H, Suda T, Oike Y. Angiopoietin-like protein 2 promotes chronic adipose tissue inflammation and obesity-related systemic insulin resistance.
Cell Metab. 2009;10:178-88. PMID:
10.1016/j.cmet.2009.08.003. PMID:
19723494.
8.
Horio E, Kadomatsu T, Miyata K, Arai Y, Hosokawa K, Doi Y, Ninomiya T, Horiguchi H, Endo M, Tabata M, Tazume H, Tian Z, Takahashi O, Terada K, Takeya M, Hao H, Hirose N, Minami T, Suda T, Kiyohara Y, Ogawa H, Kaikita K, Oike Y. Role of endothelial cell-derived angptl2 in vascular inflammation leading to endothelial dysfunction and atherosclerosis progression.
Aterioscler Tromb Vasc Biol. 2014;34:790-800.

Horio E, Kadomatsu T, Miyata K, Arai Y, Hosokawa K, Doi Y, Ninomiya T, Horiguchi H, Endo M, Tabata M, Tazume H, Tian Z, Takahashi O, Terada K, Takeya M, Hao H, Hirose N, Minami T, Suda T, Kiyohara Y, Ogawa H, Kaikita K, Oike Y. Role of endothelial cell-derived angptl2 in vascular inflammation leading to endothelial dysfunction and atherosclerosis progression.
Aterioscler Tromb Vasc Biol. 2014;34:790-800. PMID:
10.1161/atvbaha.113.303116.
9.
Sasaki Y, Ohta M, Desai D, Figueiredo JL, Whelan MC, Sugano T, Yamabi M, Yano W, Faits T, Yabusaki K, Zhang H, Mlynarchik AK, Inoue K, Mizuno K, Aikawa M.. Angiopoietin Like Protein 2 (ANGPTL2) Promotes Adipose Tissue Macrophage and T lymphocyte Accumulation and Leads to Insulin Resistance.
PLoS One. 2015;10:e0131176.

Sasaki Y, Ohta M, Desai D, Figueiredo JL, Whelan MC, Sugano T, Yamabi M, Yano W, Faits T, Yabusaki K, Zhang H, Mlynarchik AK, Inoue K, Mizuno K, Aikawa M.. Angiopoietin Like Protein 2 (ANGPTL2) Promotes Adipose Tissue Macrophage and T lymphocyte Accumulation and Leads to Insulin Resistance.
PLoS One. 2015;10:e0131176PMID:
10.1371/journal.pone.0131176. PMID:
26132105.
10.
Muramoto A, Tsushita K, Kato A, Ozaki N, Tabata M, Endo M, Oike Y, Oiso Y. Angiopoietin-like protein 2 sensitively responds to weight reduction induced by lifestyle intervention on overweight Japanese men.
Nutr Diabetes. 2011;1;e20.


Muramoto A, Tsushita K, Kato A, Ozaki N, Tabata M, Endo M, Oike Y, Oiso Y. Angiopoietin-like protein 2 sensitively responds to weight reduction induced by lifestyle intervention on overweight Japanese men.
Nutr Diabetes. 2011;1:e20PMID:
10.1038/nutd.2011.16. PMID:
23154406.
11.
Shimizu I, Walsh K. Vascular remodeling mediated by Angptl2 produced from perivascular adipose tissue.
J Mol Cell Cardiol. 2013;59:176-8.

Shimizu I, Walsh K. Vascular remodeling mediated by Angptl2 produced from perivascular adipose tissue.
J Mol Cell Cardiol. 2013;59:176-8. PMID:
10.1016/j.yjmcc.2013.03.009. PMID:
23528806.
12.
Hata J, Mukai N, Nagata M, Ohara T, Yoshida D, Kishimoto H, Shibata M, Hirakawa Y, Endo M, Ago T, Kitazono T, Oike Y, Kiyohara Y, Ninomiya T. Serum Angiopoietin-Like Protein 2 Is a Novel Risk Factor for Cardiovascular Disease in the Community: The Hisayama Study.
Arterioscler Thromb Vasc Biol. 2016;36:1686-91.

Hata J, Mukai N, Nagata M, Ohara T, Yoshida D, Kishimoto H, Shibata M, Hirakawa Y, Endo M, Ago T, Kitazono T, Oike Y, Kiyohara Y, Ninomiya T. Serum Angiopoietin-Like Protein 2 Is a Novel Risk Factor for Cardiovascular Disease in the Community: The Hisayama Study.
Arterioscler Thromb Vasc Biol. 2016;36:1686-91. PMID:
27365403.
13.
Polak JF, OŌĆÖLeary DH. Carotid intima-media thickness as surrogate for predictor CVD.
Glob Heart. 2016;11:295-312.

Polak JF, OŌĆÖLeary DH. Carotid intima-media thickness as surrogate for predictor CVD.
Glob Heart. 2016;11:295-312. PMID:
27741977.
14.
Jung CH, Lee WJ, Lee MJ, Kang YM, Jang JE, Leem J, La Lee Y, Seol SM, Yoon HK, Park JY. Association of serum angiopoietin-like protein 2 with carotid intima-media thickness in subjects with type 2 diabetes.
Cardiovasc Diabetol. 2015;14:35.


Jung CH, Lee WJ, Lee MJ, Kang YM, Jang JE, Leem J, La Lee Y, Seol SM, Yoon HK, Park JY. Association of serum angiopoietin-like protein 2 with carotid intima-media thickness in subjects with type 2 diabetes.
Cardiovasc Diabetol. 2015;14:35PMID:
10.1186/s12933-015-0198-z. PMID:
25889082.
15.
Mozos I, Malainer C, Horbańczuk J, Gug C, Stoian D, Luca CT, Atanasov AG. Inflammatory markers for arterial stiffness in cardiovascular diseases.
Front Immunol. 2017;8:1058..

Mozos I, Malainer C, Horbańczuk J, Gug C, Stoian D, Luca CT, Atanasov AG. Inflammatory markers for arterial stiffness in cardiovascular diseases.
Front Immunol. 2017;8:1058PMID:
10.3389/fimmu.2017.01058. PMID:
28912780.
16.
Miyaki A, Maeda S, Yoshizawa M, Misono M, Saito Y, Sasai H, Endo T, Nakata Y, Tanaka K, Ajisaka R. Effect of weight reduction with dietary intervention on arterial distensibility and endothelial function in obese men.
Angiology. 2009;60:351-7.

Miyaki A, Maeda S, Yoshizawa M, Misono M, Saito Y, Sasai H, Endo T, Nakata Y, Tanaka K, Ajisaka R. Effect of weight reduction with dietary intervention on arterial distensibility and endothelial function in obese men.
Angiology. 2009;60:351-7. PMID:
10.1177/0003319708325449. PMID:
19022788.
17.
Kumagai H, Zempo-Miyaki A, Yoshikawa T, Eto M, So R, Tsujimoto T, Nishiyasu T, Tanaka K, Maeda S. Which cytokine is the most related to weight loss-induced decrease in arterial stiffness in overweight and obese men?
Endocr J. 2018;65:53-61.

Kumagai H, Zempo-Miyaki A, Yoshikawa T, Eto M, So R, Tsujimoto T, Nishiyasu T, Tanaka K, Maeda S. Which cytokine is the most related to weight loss-induced decrease in arterial stiffness in overweight and obese men?
Endocr J. 2018;65:53-61. PMID:
28966223.
18.
Pase MP, Grima NA, Sarris J. The effects of dietary and nutrient interventions on arterial stiffness: a systematic review.
Am J Clin Nutr. 2011;93:446-5.


Pase MP, Grima NA, Sarris J. The effects of dietary and nutrient interventions on arterial stiffness: a systematic review.
Am J Clin Nutr. 2011;93:446-5. PMID:
10.3945/ajcn.110.002725. PMID:
21147858.
19.
Yoshikawa T, Zempo-Miyaki A, Kumagai H, Myoenzono K, So R, Tsujimoto T, Tanaka K, Maeda S. Relationships between serum free fatty acid and pulse pressure amplification in overweight/obese men: insights from exercise training and dietary modification.
J Clin Biochem Nutr. 2018;62:254-8.

Yoshikawa T, Zempo-Miyaki A, Kumagai H, Myoenzono K, So R, Tsujimoto T, Tanaka K, Maeda S. Relationships between serum free fatty acid and pulse pressure amplification in overweight/obese men: insights from exercise training and dietary modification.
J Clin Biochem Nutr. 2018;62:254-8. PMID:
10.3164/jcbn.17-103. PMID:
29892165.
20.
Tanaka K, Okura T, Shigematsu R, Nakata Y, Lee DJ, Wee SW, Yamabuki K. Target value of intraabdominal fat area for improving coronary heart disease risk factors.
Obes Res. 2004;12:695-703.

Tanaka K, Okura T, Shigematsu R, Nakata Y, Lee DJ, Wee SW, Yamabuki K. Target value of intraabdominal fat area for improving coronary heart disease risk factors.
Obes Res. 2004;12:695-703. PMID:
10.1038/oby.2004.81. PMID:
15090639.
21.
Kagawa A. The four-food-group-point-method (in Japanese). J Kagawa Nutr Univ. 1983;14:5-12.
Kagawa A. The four-food-group-point-method (in Japanese).
J Kagawa Nutr Univ. 1983;14:5-12.
22.
Shiga T, Hamaguchi T, Oshima Y, Kanai H, Hirata M, Hosoda K, Nakao K. A new simple measurement system of visceral fat accumulation by bioelectrical impedance analysis. World Congress on Medical Physics and Biomedical Engineering, September 7 - 12, 2009, Munich, Germany. Springer. 2009:338-41.
Shiga T, Hamaguchi T, Oshima Y, Kanai H, Hirata M, Hosoda K, Nakao K. A new simple measurement system of visceral fat accumulation by bioelectrical impedance analysis. World Congress on Medical Physics and Biomedical Engineering, September 7 - 12, 2009, Munich, Germany.
Springer 2009;338:41.
23.
Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker-Boudier H; European Network for Non-invasive Investigation of Large Arteries. Expert consensus document on arterial stiffness: methodological issues and clinical applications.
Eur Heart J. 2006;27:2588-605.

Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker-Boudier H. European Network for Non-invasive Investigation of Large Arteries. Expert consensus document on arterial stiffness: methodological issues and clinical applications.
Eur Heart J. 2006;27:2588-605. PMID:
10.1093/eurheartj/ehl254. PMID:
17000623.
24.
Sugawara J, Hayashi K, Yokoi T, Cortez-Cooper MY, DeVan AE, Anton MA, Tanaka H. Brachial-ankle pulse wave velocity: an index of central arterial stiffness?
J Hum Hypertens. 2005;19:401-6.


Sugawara J, Hayashi K, Yokoi T, Cortez-Cooper MY, DeVan AE, Anton MA, Tanaka H. Brachial-ankle pulse wave velocity: an index of central arterial stiffness?
J Hum Hypertens. 2005;19:401-6. PMID:
10.1038/sj.jhh.1001838. PMID:
15729378.
25.
Tanaka H, Dinenno FA, Monahan KD, Clevenger CM, DeSouza CA, Seals DR. Aging, habitual exercise, and dynamic arterial compliance.
Circulation. 2000;102:1270-75.

Tanaka H, Dinenno FA, Monahan KD, Clevenger CM, DeSouza CA, Seals DR. Aging, habitual exercise, and dynamic arterial compliance.
Circulation. 2000;102:1270-75. PMID:
10.1161/01.cir.102.11.1270. PMID:
10982542.
26.
Sone H, Yoshimura Y, Tanaka S, Iimuro S, Ohashi Y, Ito H, Seino H, Ishibashi S, Akanuma Y, Yamada N; Japan Diabetes Complications Study (JDCS) Group. Cross-sectional association between BMI, glycemic control and energy intake in Japanese patients with type 2 diabetes.
Diabetes Res Clin Pract. 2007;1:S23-9.

Sone H, Yoshimura Y, Tanaka S, Iimuro S, Ohashi Y, Ito H, Seino H, Ishibashi S, Akanuma Y, Yamada N. Japan Diabetes Complications Study (JDCS) Group. Cross-sectional association between BMI, glycemic control and energy intake in Japanese patients with type 2 diabetes.
Diabetes Res Clin Pract. 2007;1:S23-9.
27.
Takahashi K, Yoshimura Y, Kaimoto T, Kunji D, Komatsu T, Yamamoto S. Validation of a food frequency questionnaire based on food groups for estimating individual nutrient intake.
Japaness J Nutr Diet. 2001;59:221-32.

Takahashi K, Yoshimura Y, Kaimoto T, Kunji D, Komatsu T, Yamamoto S. Validation of a food frequency questionnaire based on food groups for estimating individual nutrient intake.
Japaness J Nutr Diet. 2001;59:221-32. PMID:
10.5264/eiyogakuzashi.59.221.
28.
Mozos I, Malainer C, Horbańczuk J, Gug C, Stoian D, Luca CT, Atanasov AG. Inflammatory Markers for Arterial Stiffness in Cardiovascular Diseases.
Front Immunol. 2017;8:1058.

Mozos I, Malainer C, Horbańczuk J, Gug C, Stoian D, Luca CT, Atanasov AG. Inflammatory Markers for Arterial Stiffness in Cardiovascular Diseases.
Front Immunol. 2017;8:1058PMID:
10.3389/fimmu.2017.01058. PMID:
28912780.
29.
Desjardins MP, Thorin-Trescases N, Sidib├® A, Fortier C, De Serres SA, Larivi├©re R, Thorin E, Agharazii M. Levels of Angiopoietin-Like-2 Are Positively Associated With Aorotic Stiffness and Mortality After Kidney Transplantation.
Am J Hypertens. 2017;30:409-16.


Desjardins MP, Thorin-Trescases N, Sidib├® A, Fortier C, De Serres SA, Larivi├©re R, Thorin E, Agharazii M. Levels of Angiopoietin-Like-2 Are Positively Associated With Aorotic Stiffness and Mortality After Kidney Transplantation.
Am J Hypertens. 2017;30:409-16. PMID:
10.1093/ajh/hpw208. PMID:
28158589.