INTRODUCTION
Increased occurrence of lipid species such as triglyceride, diacylglycerol, and ceramide in skeletal muscle, reportedly increases stored fat mass on consumption of high fat diet, resulting in impairment of insulin signaling pathway and glucose transfer system1. Over-intake of a high fat diet leads to metabolic syndrome, and excessive eating of carbohydrates and proteins also affects insulin resistance2,3. Insulin resistance induced by a high fat diet involves decreased protein levels of insulin signaling pathway factors, such as insulin receptor (IR), phosphoinositide 3-kinase (PI3K), and Akt4,5.
Dietary overindulgence activates mTORC1 that is stimulated by mTOR, inhibits the activity of PI3K through phosphorylation of ribosomal S6 protein kinase 1 (S6K1) to decompose amino acid6, and results in preventing phosphorylation of tyrosine and suppression of insulin receptor substrate 1 (IRS-1)7-9. Inhibition of IRS-1 through reduction of tyrosine phosphorylation and S6K1 phosphorylation also results in the activation of mTORC110,11. Therefore, mTORC1 activation in the skeletal muscle could induce insulin resistance. However, the role of mTORC2 associated with insulin signaling is currently unknown.
Endurance training and regular exercise have positive effects on insulin action and mTOR signaling. A serine-threonine protein kinase (AKT) is activated by PI3K pathway through exercise in the form of external stimulation of the cell12, and regulates cellular organization and hypertrophy13,14. However, the effect of exercise and/or dietary change on the metabolic pathways associated with continuous high fat diet-induced obesity is currently unclear. In addition, the precise mechanisms by which exercise and/or dietary change affect upstream and downstream mTOR signaling pathway related in insulin resistance is currently unknown.
Therefore, the objective of this study was to investigate the effect of exercise and dietary change on obesity and insulin resistance and mTOR signaling protein levels in skeletal muscle of obese rats induced by high fat diet for 15 weeks.
METHODS
Experimental animals and treatments
Sixty male Sprague Dawley (SD, 4 weeks old) rats were housed in cages and fed freely with standard rat chow and water (Daehan Biolink, Korea). Three or 4 rats were housed in each cage, and maintained under standardized conditions in an animal facility (Laboratory of animals, College of Medicine, Dong-A University), with a room temperature of 22 ± 1.5°C, 50 ~ 60 % relative humidity, and a 12 hour light/dark cycle. All rats were cared for during the entire period of experimentation in accordance with the Guidelines of Animal Experiments recommended by the Institutional Animal Care and Use Committee. After 1 week of adaptation maintenance, rats were randomly divided into two groups to induce obesity by high fat diet for 15 weeks: a normal diet (CO) group (12 % fat; Donga SF, Korea, n = 20), and a high fat diet (HF) group (40 % fat; AIN-76A; Jungang Lab Animal, Inc, Korea, n = 40). Body weight was measured every week during the entire experimental period.
Exercise program
After inducing obesity, rats were randomly subdivided into the CO, COT (CO + training), HF, HFT (HF + training), HFND (dietary change to normal diet), and HFNDT (HFND + training) groups (10 rats per group). Rats in the exercise training groups were put on a treadmill for 40 min once a day, 5 times a week, for 8 weeks. Exercise intensity consisted of 5 m/min (5 min), 12 m/min (5 min), and 18m/min (20 min) at 0% slope for weeks 1 to 4 (low intensity). During weeks 5 to 8, exercise intensity was increased to 10 m/min (5 min), 16 m/min (5 min), and 22 m/min (30 min) at the same slope (moderate intensity)15.
Blood and tissue samplings
To exclude the temporary effects of treadmill exercise, sacrifice was conducted after 48 hours from the last exercise session. After complete anesthesia (ethyl ether), blood samples (5 ml) from the abdominal vena cava were obtained in syringes. Plasma was collected by centrifugation of heparinized blood at 3000 rpm for 15 min. Soleus muscle were removed and stored at -70°C until analysis.
Lipid profiles
Plasma total cholesterol (TC) and triglyceride (TG) levels were analyzed with rat TC and TG kits (Asan Pharmaceutical, Korea). High density lipoprotein cholesterol (HDL-c) level was analyzed with HDL-c kits (Shinyang Diagnostics, Korea) and Low density lipoprotein cholesterol (LDL-c) was calculated with the following equation: LDL-c = TC - (HDL-c + TG/5)16. Plasma insulin level was analyzed with a rat insulin ELISA kit (Shibayagi Co. Ltd, Japan) according to the manufacturer’s instructions. Blood glucose level was estimated using a GlucoDr glucometer (Allmedicus, Korea). Insulin resistance index (IRI) was assessed by homeostasis model assessment estimate of insulin resistance (HOMA-IR) as follows:
IRI = Fasting insulin (µIU/mL) X Fasting glucose (mg/dL) / 405
Western blot
To extract protein from the soleus muscle, tissues were homogenized after adding a solution containing 150 mM NaCl, 5 mM EDTA, 50 mM Tri-HCl (pH 8.0), 1 %-NP 40, 1 mM aprotinin, 0.1 mM leupeptin, and 1 mM pepstatin. The solution was centrifuged for 30 minutes at 13,000 rpm. Supernatants were collected and assayed for protein content prior to storage at -70°C. Protein samples were mixed with Laemmli sample buffer (LSB) and placed in a boiling water bath for 5 min. Proteins were resolved by 10, 12 or 15 % SDS-polyacrylamide gel electrophoresis (SDS-PAGE; each loaded with same μg of total protein per lane), and transferred to nitrocellulose membranes. Proteins on the membranes were blocked in 5 % skim milk in phosphate - buffered saline (PBS) (NaCl 8 g, KCl 0.2 g, Na2HPO4 1.44 g, KH2PO4 0.24 g, pH 7.4). Thereafter, protein membranes were incubated with the following primary antibodies: IRS-1, 2 (#2382, #4502, Cell Signaling), Akt (#9272, Cell Signaling), p-Akt (#4060, Cell Signaling), mTOR (#2972, Cell Signaling), mTORC1 (#2280, Cell Signaling), mTORC2 (#2114, Cell Signaling), PI3K (#4255, Cell Signaling), S6K1 (#9202, Cell Signaling) for one hour, and washed thrice (15 min each) in a PBS solution containing 0.1 % tween 20. Washed membrane was then treated with secondary antibody (goat anti mouse or rabbit IgG) conjugated with horseradish peroxidase (HRP). Immune-reactive bands were developed on Kodak film. The relative strengths of bands were quantitated by densitometry (Sci - Scan, UUSB).
Data analysis
The change in body weight induced by high fat diet was analyzed by independent t-test and ANOVA. One-way ANOVA and Duncan’s post-hoc analysis were performed for any intergroup difference observed. All data were tested for normal distribution using the Shapiro-Wilk test. All data were analyzed using SPSS Software Version 21.0 for Windows (SPSS Inc. Chicago. IL). Data were expressed as mean ± standard error (SE). Statistical significance was defined as a p value < 0.05.
RESULTS
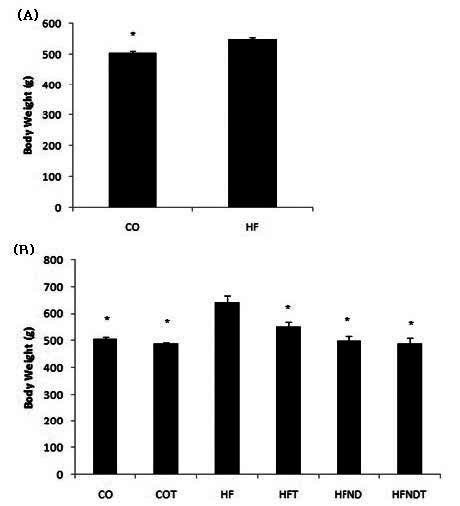
After 15 weeks of a high fat diet, body weight in HF group was significantly higher, as compared to the CO group (p <.05) (Figure 1-A). After 8 weeks of exercise and dietary change, body weight in the HFT, HFND, and HFNDT groups was significantly lower than in the HF group (p <.05) (Fig. 1-B).
Figure 1.
mean±SE, (A) Changes of body weight after 15 weeks of high-fat diet, (B) Changes of body weight after exercise and dietary change for 8 weeks. *p<0.05 vs HF. CO; normal diet group, COT; normal diet + training group, HF; high fat diet group, HFT; high fat diet + training group, HFND; dietary change group, HFNDT; dietary change + training group.
TC was significantly higher in the HF group than in the COT, HFT, HFND, and HFNDT groups (p <.05). TG was higher in the HF group, as compared with all other groups (p <.05). HDL-c level was significantly lower in the HF group than in the COT and HFNDT groups (p <.05). LDL-c level was significantly higher in the HF group than in the HFNDT group (p <.05). Glucose level was significantly higher in the HF group than in the CO, COT, and HFNDT groups (p <.05). Insulin and HOMA-IR levels were significantly higher in the HF group, as compared with all other groups (p <.05) (Table 1).
Table 1.
Change of lipid profiles after 8 weeks treatment
After 8 weeks of exercise and dietary change, muscle protein level of IRS-1, IRS-2, p-Akt, and mTOR in the HF group were significantly lower, as compared with all other groups (p <.05). Also, muscle protein level of IRS-2, p-Akt and mTOR in the CO group were significantly higher, as compared with the COT group (p <.05). However, Akt protein level was not significantly different, as compared with all other groups (Fig. 2).
Figure 2.
mean±SE, *p<0.05 vs HF, #p<0.05 vs CO. CO; normal diet group, COT; normal diet + training group, HF; high fat diet group, HFT; high fat diet + training group, HFND; dietary change group, HFNDT; dietary change + training group.

The mTORC1 protein level was significantly higher in the HF group than in all other groups (p <.05). In addition, mTORC1 protein level were significantly lower in the HFND and HFNDT groups, as compared with the HFT group (p <.05). mTORC2 protein levels of were significantly higher in the CO, COT, and HFNDT groups, as compared with the HF group (p <.05). The PI3K protein level was significantly lower in the HF group, as compared with all other groups (p <.05). In addition, the HFND and HFNDT groups showed significantly higher levels of PI3K compared with the HFT group (p <.05). S6K1 protein level was significantly higher in the HF group, as compared with all other groups (p <.05). In addition, the HFND and HFNDT groups showed significantly lower levels of S6K1, as compared with the HFT group (p <.05) (Fig. 3).
DISCUSSION
Regular exercise and dietary change can be effective for regulation of body weight and insulin resistance. Previous studies have reported that regular exercise has positive effects on insulin resistance in skeletal muscle via mTOR signaling pathway. We aimed to determine the effect of exercise and dietary change on biochemical changes of mTOR signaling pathway, in case of obesity induced by continuous consumption of high fat diet. Therefore, we evaluated the insulin resistance and mTOR signaling protein levels in skeletal muscle of high fat diet induced obese rats after 8 weeks of regular exercise and dietary change.
Excessive intake of carbohydrates, proteins, and fats negatively affects insulin action in skeletal muscle17,18, partly via glucose metabolism1. High fat diet induced obesity reportedly causes insulin resistance and leptin resistance in peripheral tissues19,20. A member of the mTOR signaling pathway, mTORC1, is known to negatively control insulin levels through inhibition of IRS-19. Although mTORC2 prevents the activation of S6K121, the effect of mTORC2 on insulin activity is currently unclear. However, mTORC2 controls the activation and phosphorylation of Akt, and reduced mTORC2 in skeletal muscle leads to decreased glucose uptake via insulin induction22. Therefore, mTORC2 and Akt may play important roles in the activation of insulin that is essential for glucose homeostasis. In this study, we confirmed that protein levels of mTORC2, PI3K, and p-Akt were significantly decreased by high fat diet. In addition, high fat diet induced obese rats had high protein levels of mTORC1 and S6K1, but low protein levels of IRS-1 and IRS-2. Therefore, high fat diet-induced metabolic pathway showed a negative effect on insulin action in skeletal muscle.
Exercise increases metabolism in skeletal muscle by improving insulin action and glucose uptake23,24, is suggested as the most effective method for the treatment of insulin resistance in skeletal muscle25. A previous study reported that exercise has a positive effect on insulin resistance19 and leptin resistance20 that is mediated by improvements in glucose metabolism. Exercise-induced mTOR complex activity suggests that phosphatidic acid is stabilized by the formation of mTOR complexes, therefore regulating mTORC1 in response to metabolic stimulation by nutrients and growth factors26,27. A previous study reported that acute exercise increases activation of mTOR for a few hours28. In addition, both aerobic exercise through the various muscle contractions29 and high-intensity resistance exercise30 activate mTOR. Previously, a report indicated that regular exercise regulates the insulin signaling pathway via PI3K activation31. Chibalin et al., demonstrated that swimming exercise changes the level of IR in Wistar rats32; however, this is unlikely to be the sole explanation for the increased metabolic response to insulin, because insulin stimulates glucose transport activity, as evidenced by a dramatic increase in GLUT4 protein expression after just one day of exercise. Another study showed that 6 weeks exercise improved skeletal muscle insulin resistance without reduced mTOR/S6K1 signaling pathway33. However, the body weight of rats showed no increase in response to 59% high-fat diet for 6 weeks, and exercise for 6 weeks33. The discrepancy in results of previous study as compared to the present study, is possibly due to insufficient exercise duration, and differences in fat composition.
Dietary intervention is another effective method for the treatment of insulin resistance. Many studies report that caloric restriction increases insulin sensitivity34,35, and this result might be due to the reduced body weight and fat mass36. In addition, previous studies conducted a combination of exercise and/or dietary restriction to analyze changes in glucose metabolism37,38. Ross et al., reported physical activity without caloric restriction increased weight loss, and substantially reduces insulin resistance37; and Larson-Meyer et al., reported caloric restriction alone or with exercise ameliorated insulin resistance36. In this study, we confirmed that regular exercise and/or dietary change reduces the level of mTORC1 but activates mTORC2, suggesting that mTORC1 and mTORC2 may act to ameliorate obesity and insulin resistance.
In the present study, we show a negative effect of the mTOR signaling pathway in obese rats induced by high fat diet. However, regular exercise and dietary change directly brought about improvements of glucose metabolism and insulin level. In addition, our study demonstrated that regular exercise decreases mTORC1 levels, and the exercise, dietary change, and combination of treatments ameliorate obesity and insulin resistance in skeletal muscle via increasing mTORC2 and p-Akt protein levels.
In summary, regular exercise and/or dietary change ameliorates obesity and insulin resistance via regulating mTORC1 and mTORC2 protein. Therefore, despite the negative impact of continuous high fat diet intake, regular exercise and dietary change results in a positive effect on insulin resistance and mTOR signaling protein levels.









