DHEA administration has limited effect onintestinal Ca absorption in ovariectomized rats
Article information
Abstract
[Purpose]
The effect of dehydroepiandrosterone (DHEA) administration on intestinal Calcium (Ca) absorption in estrogen deficiency state has not been studied yet. We examined the bone mineral content (BMC) of lumbar spine and Ca balance such as intestinal Ca absorption and Ca accumulation in ovariectomized (OVX) rats after 8 weeks of DHEA administration.
[Methods]
Seventeen female Sprague-Dawley rats, 6 weeks old, were randomized into two groups: OVX control rats (OC, n = 8) and OVX rats with DHEA treatment (OD, n = 9). DHEA was administered to the OD group intraperitoneally at 20 mg DHEA/kg body weight for 8 weeks while the OC group was treated with vehicle only.
[Results]
The BMC normalized by body weight of the lumbar spine (trabecular-abundant region) in the OD group was found to be significantly higher compared to that in the OC group. The femoral wet weight normalized by body weight in the OD group was significantly higher compared to that in the OC group. The intestinal Ca absorption, rate of intestinal Ca absorption, Ca accumulation, and rate of Ca accumulation decreased from the 4th and 5th of the experimental diet period to the end of the experimental period, but interaction of time and group was not observed. In both periods, all parameters did not differ between the groups.
[Conclusion]
The present study confirmed the positive effect of DHEA on trabecular bone mass in ovariectomized rats. On the other hand, DHEA administration might have limited the impact on intestinal Ca absorption in estrogen deficiency state.
INTRODUCTION
Osteoporosis is one of the most serious public health problems for elderly people and also a major cause of the bedridden state. This condition/disease has threatened the quality of life in old age and increased medical costs [1,2]. In particular, the potential for developing osteoporosis increases dramatically after menopause in females, and estrogen deficiency causes rapid bone loss of trabecular regions in hip or lumbar spine. Estrogen deficiency is also associated with decrease in intestinal Ca absorption which results in further acceleration of bone loss [3,4]. Although it is well known that decrease in intestinal Ca absorption is attributable to estrogen deficiency, it remains unclear what actions other gonadal hormones have for the association between bone mass and intestinal Ca absorption in the estrogen deficiency state.
Dehydroepiandrosterone (DHEA), secreted mainly from the adrenal gland and ovary, plays a critical physiological role for maintaining steroidogenesis by being used as the available precursor converted to testosterone and estrogens in various peripheral tissues such as bones, liver, brain, and skeletal muscles [5]. DHEA concentration in the blood decreases the following ovariectomy in animals [6,7]. On the other hand, DHEA replacement improves bone mineral density (BMD), especially a trabecular site, in the ovariectomized (OVX) animals [6-8]. We previously observed that DHEA replacement increased E2 (Estradiol) centration in the blood [8]. The presence of estrogen receptors in the intestinal mucosa and estrogen stimulates intestinal calcium absorption via an estrogen receptor [9]. However, to our knowledge, the effect of DHEA administration on intestinal Ca absorption in estrogen deficiency state has not been studied yet.
Accordingly, we hypothesized that DHEA administration would increase intestinal Ca absorption via increasing E2 concentration in the blood and prevent trabecular bone loss caused by estrogen deficiency. In the present study, we examined bone mass in a trabecular abundant site of lumbar spine and Ca balance such as intestinal Ca absorption and Ca accumulation in OVX rats after 8 weeks of DHEA administration.
METHODS
Experimental animals and feeding protocol
Seventeen female Sprague-Dawley rats, 6 weeks old, were surgically ovariectomized and randomized into two groups: OVX control rats (OC, n = 8) and OVX rats with DHEA treatment (OD, n = 9). This study was a part of Park et al.’ study [8]. Briefly, all rats were fed a diet with 1.05% calcium and 1.01% phosphate purchased from CLEA Japan (CE-2, CLEA Japan, Inc., Japan) during the experiment. DHEA dissolved in sesame oil was administered to the OD group intraperitoneally at 20 mg DHEA/kg body weight for 8 weeks beginning one week after ovariectomy while the OC group was treated with vehicle only (sesame oil, 0.5 ml). Rats were not treated with DHEA or vehicle every fourth day (i.e., they were treated for 3 consecutive days). No inflammation was observed in DHEA-treated rats of the present study. The rats were kept in individual cages (15 × 25 × 19.5 cm3) and allowed access to food and distilled water ad libitum. Food consumption and body weight gain were measured every second day. The room temperature was maintained at 24 ± 1°C, and the humidity at 50 ± 5%. Fluorescent lights were turned on from 8:00 a.m. to 8:00 p.m. Animal care and experimental procedures were approved by the Animal Experimental Committee of the University of Tsukuba.
Measurement of Bone Mineral Content (BMC) and Bone Mineral Density (BMD)
The lumbar spine, left, and right tibiae of each rat were isolated by dissection, and all the muscle and connective tissue was carefully removed. Thereafter, BMC and BMD value for the L3-L6 lumbar spine were measured by Dual - energy X-ray absorptiometry (DXA; Aloka DCS-600R instrument) [10]. In the present study, we used young OVX rats whose growth of bone is considerably influenced by body mass. Therefore, we normalized body weight for BMC to evaluate the effect of DHEA on bone mass [11].
Femoral diameters, weights, and breaking force
At the each dissection, femur samples were isolated after killing by exsanguinations. After the adhering connective tissues had been trimmed off, the femoral wet weight was measured. Thereafter, the femoral length, long width, and short width were measured with a caliper. Length was measured from the proximal tip of the femur head to the distal tip of the medial condyle. The mediolateral (long width) and anteroposterior (short width) dimensions were measured at the midpoint of the femur diaphysis. The bone strength of the middle diaphysis of the femur was then tested by measuring the mechanical strength, breaking force with an Iio DYN-1255 instruments. The force necessary to produce a break at the center of the femur was measured under the following conditions; the sample space was 1.0 cm, the plunger speed was 100.0 mm/min, the load range was 50.0 kg, and the chart speed was 120.0 cm/min [12].
Ca balance study
In this study, two balance studies were carried out to determine the rate of intestinal Ca absorption and Ca accumulation. Animals were placed in individual metabolic cages (24 × 20 × 18 cm3). The first phase was carried out on the 4th and 5th day after starting the experimental diets period (Metabolic cage phase 1: MC 1). The next phase (MC 2) was carried out at the end of the experimental period. At each phase, feces and urine were collected over two 24-h periods. Urine was collected under acidic conditions by using 2ml 2N hydrochloric acid, thus preventing Ca precipitate and putrefaction. All urine was centrifuged at 2,500 rpm for 15 min to eliminate refuse. In the fecal determination, all daily feces were burnt to ash at 550-600℃ for approximately 18 hours, and the resulting ash was dissolved in 1N nitric acid. The level of serum Ca was measured by the Inductively Coupled Plasma Atomic Emission Spectroscopy (ICAP-AES - 575 v Nippon Jarrell-Ash). Intestinal Ca absorption and Ca accumulation were calculated using the amount of Ca intake, the fecal Ca excretion and the urinary Ca excretion.
Statistics
All the data are expressed as mean ± SE. Statistical significance (p < 0.05) was determined using an unpaired Student’s t-test between groups. Two-way analysis of variance (ANOVA) was used for determining the effects of time and group on intestinal Ca absorption and accumulation. Statistical analysis was carried out by one-way ANOVA followed by Fisher`s F-test for multiple comparisons among four points in intestinal Ca absorption and accumulation. Statistical analysis was performed using SPSS for Windows (version 20.0 J; SPSS Inc., Chicago, IL, USA). this paragraph is good)
RESULTS
The body weight gain, food intake, and food efficiency are presented in Table 1. The initial body weight did not differ between the groups. The final body weight and the body weight gain were significantly lower in the OD group than in the OC group. The food intake did not differ between the groups.
The BMC normalized by body weight of the lumbar spine (trabecular-abundant region) in the OD group was found to be significantly higher compared to that in the OC group. To eliminate the effect of body weight on growing bone, we normalized body weight for BMC for evaluating the effect of DHEA on bone mass. The BMD of the lumbar spine in the OD group tended to be higher compared to that in the OC group, but it did reach statistically significant levels.
The femoral wet weight normalized by body weight in the OD group was found to be significantly higher compared to that in the OC group, but the long and short width and length of femur did not differ between the groups (Table 2). The breaking force at femoral diaphysis site (cortical boneabundant) did not differ between the groups (Table 2).
The intestinal Ca absorption and accumulation are shown in Fig. 2. The intestinal Ca absorption, rate of intestinal Ca absorption, Ca accumulation, and rate of Ca accumulation decreased from MC 1 (the 4th and 5th of the experimental diet period) to MC 2 (the end of the experimental period), but the interaction of time and group was not observed. In both MC1 and MC2, all parameters did not differ between the groups. (Other than some article issues, this section is also solid.)
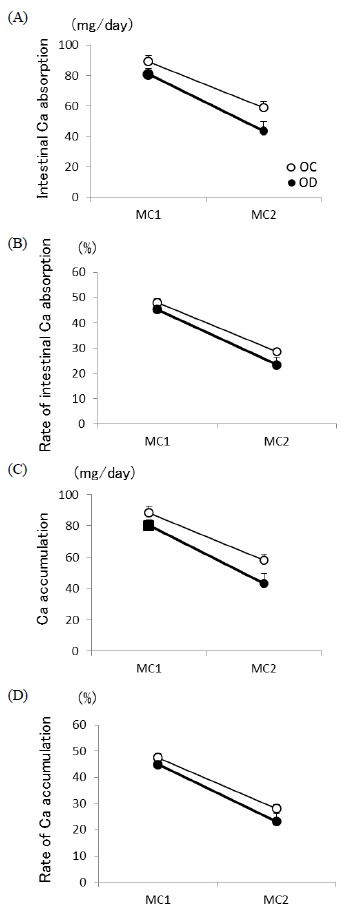
The intestinal Ca absorption (A), rate of intestinal Ca absorption (B), Ca accumulation (C), and rate of Ca accumulation (d). The first phase was carried out on the 4th and 5th day after starting the experimental diets period (Metabolic cage phase 1: MC 1). The next phase (MC 2) was carried out at the end of the experimental period. At each phase, feces and urine were collected over two 24-h periods. OVX control rats (OC, n = 8) and OVX rats with DHEA treatment (OD, n = 9). ** p < 0.01 vs. OC. Values are expressed as the mean ± SE.
DISCUSSION
The present study demonstrated that DHEA administration increased the bone mass of lumbar spine in ovariectomized rats. On the other hand, DHEA administration did not show any effect on intestinal Ca absorption.
We found that the bone mass of lumbar spine (trabecular abundant site) was increased after 8 weeks of DHEA administration. This result is similar to the result of our previous study [8] and Turner et al.’s study [6] which showed increase in trabecular bone mass of tibia. DHEA particularly may have a positive effect on trabecular bone mass but not in cortical areas in the diaphysis region [6,8]. The reason for this site-specific effect remains unclear yet, but our result further supported that DHEA administration may be effective for preventing the reduction of trabecular abundant bones at which fracture frequently occurs for postmenopausal women with osteoporosis.
In the present study, femoral weight normalized by body weight was higher in rats treated with DHEA than in control rats. The reason for the higher femoral weight would be supported by Martel et al.’ study that the total BMC of femur increased by DHEA administration in ovariectomized rats [13]. Also, we measured the femoral strength. To our knowledge, no studies have examined the effect of DHEA treatment on the bone strength of diaphysis site. However, we did not observe any effect of DHEA on the bone strength at the midpoint of the femur diaphysis. The lack of effect of DHEA on the bone strength might be partly influenced by the non-effect of DHEA on the bone mass at the diaphysial area [6,8].
Our previous study showed that E2 concentration in the blood was higher in ovariectomized rats treated with DHEA than in ovariectomized control rats [6]. Although evidence of a direct effect of estrogen promoting intestinal Ca absorption has been reported [4,9], our results revealed that DEHA administration may have no effect on intestinal Ca absorption. As reason for the lack of DHEA effect, intestinal Ca absorption might have increased by DHEA administration in an earlier phase during 8 weeks of the experimental period. Ca accumulation at the end point of the present experiment did not differ between the groups, meaning that the effect of DHEA on bone had already reached its ceiling peak level. As another possible reason for the lack of DHEA effect, we used 1.05% Ca diet which is an enough Ca amount for maintaining the bone health of ovariectomized rats. Some studies related to examining intestinal Ca absorption used to use 0.3-0.5% Ca diet condition [14] which can lead to bone loss and induce higher Ca absorption.
As the result of our previous study, the serum DHEA concentration was approximately 9-fold higher in DHEA-treated rats than in control rats [6]. Thus, we cautiously assumed that Ca absorption would have increased if we treated lower dose of DHEA than that in the present study. Therefore, future research that focuses on the dose-dependent effect of DHEA administration is necessary. In addition, we observed that DHEA administration increased BMC normalized body weight even though it did not increase BMD. During the growing phase, BMD sometimes may not precisely reflect the alteration of bone mass since growth of bone is considerably influenced by body mass. Because BMD is calculated by dividing the BMC with area (cm2), the BMD measurement by DXA did not provide the details of bone parameters such as cortical width and periosteal circumferences. Thus, the parameters should be examined in detail by using the methods of pQCT, μCT, or bone morphometry in future studies, especially when using animals in the growing phase.
CONCLUSIONS
The present study confirmed the positive effect of DHEA on trabecular bone mass of lumbar spine in ovariectomized rats. On the other hand, DHEA administration did not affect intestinal Ca absorption. We suggest that DHEA administration might have limited impact on intestinal Ca absorption in estrogen deficiency state.
Acknowledgements
The authors are grateful to all members of the Exercise and Nutrition Laboratory at the University of Tsukuba for their kind cooperation in the anatomy work. J.H.P was supported by the SMART Research Professor Program of Konkuk University.


