Effects of hippotherapy on brain function, BDNF level, and physical fitness in children with ADHD
Article information
Abstract
[Purpose]
The purpose of this study was to examine the effects of hippotherapy on brain function and levels of blood-derived neurotrophic factor (BDNF) in children with attention deficit and/or hyperactivity disorder (ADHD).
[Methods]
The hippotherapy group (HRG) included twenty children with ADHD and the control group (CG) included 19 children. All participants’ physical fitness, fMRI brain scans, and blood BDNF levels were measured at baseline and after 32 weeks of participating in hippotherapy.
[Results]
After 32 weeks of participating in hippotherapy, the body fat of the HRG was significantly decreased (-1.12 ± 4.20%) and the body fat of the CG was increased (2.38 ± 6.35%) (p=0.049). There was no significant difference of physical fitness in both groups (p>0.05). Although there was a higher decrease in the activated insular area in the HRG (-1.59 ± 0.99) than in the CG (-1.14 ± 1.41), there was no significant difference between the two groups (p>0.05) Also, there was a higher increase in the activated cerebellum area in the HRG (1.97 ± 1.45) than in the CG (1.92 ± 1.81). However, there was no significant difference between the two groups (p>0.05). BDNF levels showed an increased tendency in the HRG (166.29 ± 277.52pg) compared to the CG (21.13 ± 686.33pg); otherwise, there was not any significant difference in these blood levels between the two groups (p>0.05). It can be assumed that big individual differences in the level of ADHD in the study participants might not cause any significant results, although there might be positive changes in the brain function of children with ADHD.
[Conclusion]
Therefore, this study suggests that hippotherapy training would need to be modified and developed to increase the efficacy of hippotherapy in children with ADHD.
INTRODUCTION
Attention deficit or/and hyperactivity disorder (ADHD) mainly appears in pre-school children. Children with ADHD usually demonstrate hyperactivity, impulsivity, aggression, and simultaneously, they achieve limited academic results and ignore social rules due to attention deficiency [1,2]. ADHD morbidity has been reported to be 3-20% on average and there are some variations of this result across the body of research [3,4]. The neurophysiological causes of ADHD have been reported; these functional disorders in the right side frontal lobe and dorsal side frontal lobe may evoke decreased functional ability in personal relations and coordination [5,6]. In particular, hyperactivity, attention deficiency, impulsivity, and aggression in school age children can still be persisted into adulthood and thus, earlier intervention to relieve ADHD is urgently required to ensure successful social adaptation and social relations [7-9].
Generally, central nervous system (CNS) stimulant drugs, such as methylphenidate or amphetamine, are recommended as the most effective medicine for the management of ADHD. However, these drugs temporarily improve ADHD symptoms and do not perfectly result in eradicating the condition. Therefore, these drugs are not an appropriate treatment for curing the fundamental causes of ADHD [10,11]. To make up for the weak points of ADHD treatment, various intervention studies have been conducted to overcome the frontal lobe malfunction; these studies have explored the effect of participating in physical activity for at least 20 minutes and doing so regularly [12-14].
Previous studies examined how participating in therapeutic horse-riding and/or hippotherapy as well as in general physical activity might improve social role behavior, quality of life, and motor function in children with ADHD. This suggests the need for various and in-depth studies to improve ADHD treatment [15,16]. However, few studies have applied hippotherapy in ADHD children in Korea and thus, such a local study is necessary.
Until now, the best well known part of hippotherapy is its effective treatment for improving gross motor function. It has been reported that when children with cerebral palsy (a gross motor function disorder) participated in hippotherapy 1-2 times per week, their neuromuscular and psychological functions were improved [17,18]. Accordingly, we can expect that hippotherapy for children with ADHD may induce positive changes in brain function and brain-derived neurotrophic factor (BDNF). Therefore, the purpose of this study was to examine the effects of hippotherapy on brain function and basal BDNF changes in children with ADHD.
METHODS
Participants
ADHD participants in this study were screened from J and K university psychiatric patients and 20 children diagnosed as ADHD patients were selected. Twenty ADHD children who participated in a horse-riding rehabilitation therapy program were selected for inclusion in the hippotherapy group (HRG) and 19 children who participated in a physical rehabilitation activity program were selected for inclusion in the control group (CG). Informed consent from all participants and their parents was collected and their basic demographical information was acquired (See Table 1). A preliminary report of their physical and mental disease history, which might affect their functional MRI (fMRI) result, was compiled before the participants engaged in this study. Additionally, all participants were screened for physical and mental health using the Kiddie-Schedule for Affective Disorders and Schizophrenia. To evaluate the effectiveness of hippotherapy, all participants' physical characteristics, muscle and cardiorespiratory fitness, fMRI scan, and BDNF levels were measured at baseline and 32 weeks after undergoing a program of hippotherapy (pre and post).
Study design
This study was a randomized block research design that investigated the effects of hippotherapy on brain function in children with ADHD over 32 weeks (1 time per week).
Hippotherapy training
Hippotherapy training was conducted and combined with the horse-riding rehabilitation program provided once a week by the K Area Boucher Business Program for 32 weeks. For the first 5 weeks of hippotherapy training, all participants experienced and learned to walk, trot, canter, and gallop and for the next 5 weeks, all participants did a half seat posture training exercise. On horse-back, they learned how to walk and trot for the first 23 weeks and canter between the 23rd week until the 28th week. For the last four weeks, all participants could trot independently. All training included a 15-minute warm-up, a 35-minute horse ride, and a 10-minute cool down. To secure all participants’ safety, one leader and two assistants walking on either side of the horse accompanied each participant.
Measurement and analysis items
Before and after participating in hippotherapy, participants’ height, weight, and body mass index (BMI), as well as their physical strength and cardiorespiratory fitness were measured. Activated brain function and BDNF levels were estimated by fMRI.
Physical characteristics
The BMI of all participants was calculated using a BMI formula [weight(kg) / height(m)2]. Body fat was estimated using dual energy X-ray absorptiometry (DEXA; HV-PS 7681, Lunar, USA).
Muscular and cardiorespiratory fitness
Knee extension strength (angular velocity at 60°, dominant leg) represented muscular strength. This was measured by the isokinetic machine (Isomed 2000, Germany) and a standing long jump was conducted. A graded treadmill test was also conducted to identify cardiorespiratory (VO2max) changes induced by an increase in regular physical activity.
Functional MRI (fMRI)
To confirm the effect of hippotherapy on the brain, 8 participants from 2 groups were randomly selected. Subsequently, an fMRI scan of the interior insular and cerebellar area was conducted to compare the results before and after hippotherapy. A fMRI was used by the 3.0 Tesla GE MRI scanner (General Electric Medical Systems, Milwaukee, WI) and a T-1 image was scanned to collect 240 sagittal images of the brain using a 3D spoiled gradient echo pulse sequence (SPGR). When the IR-prepared 3D SPGR sequence was performed, Zerofill Interpolation Processing (ZIP) was used to reconstitute images. To identify abnormalities in the brain structure, a T-2 image was also used and all fMRI scans used Quadrature type head coils.
Blood BDNF
To estimate BDNF, blood was taken and analyzed using human enzyme-linked immunosorbant assay kits (AB Frontier). Blood from the forearm vein was centrifuged by 1000 Xg (RCF) for 15 minutes and 0.1 ml of centrifuged serum was analyzed using the Microplate model 680 (Bio-Rad, USA) with enzyme-linked immunosorbent assay (ELISA) method. Concentration values were obtained by the measured optical density (O.D) and by calculating density.
Data analysis
SPSS 18.0 for Window (Chicago, IL, USA) was used to process this study data and the collected data was tested using descriptive statistics (mean and standard deviation). A between group difference test was conducted using an independent t-test that considers the delta t value (pre and post) to correct the type II error according to the baseline difference between the two groups. In addition, a significant fMRI result comparison was conducted; this comparison considered the corrected p-value to be below 0.05. The fMRI result comparison (between the groups, test conditions, and test condition x between groups) was conducted using a t-test that employed the Brain Voyager Program, which considers age and gender. The statistical significance was set at p<.05.
RESULTS
There was no significant difference in physical fitness, fMRI scans, and BDNF levels between the two groups before hippotherapy (see Table 2). After 32 weeks of hippotherapy, body fat in ADHD children had changed; body fat of the HRG had decreased (-1.12 ± 4.20%) and the CG had increased (2.38 ± 6.35%), which represented a significant difference between two groups (p = 0.049) (See Fig. 1).
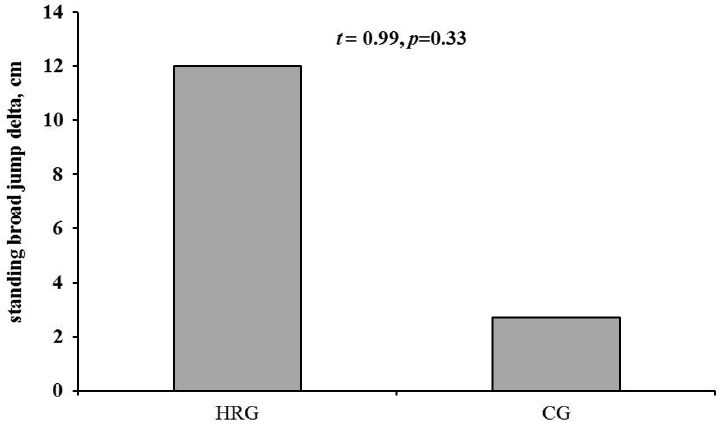
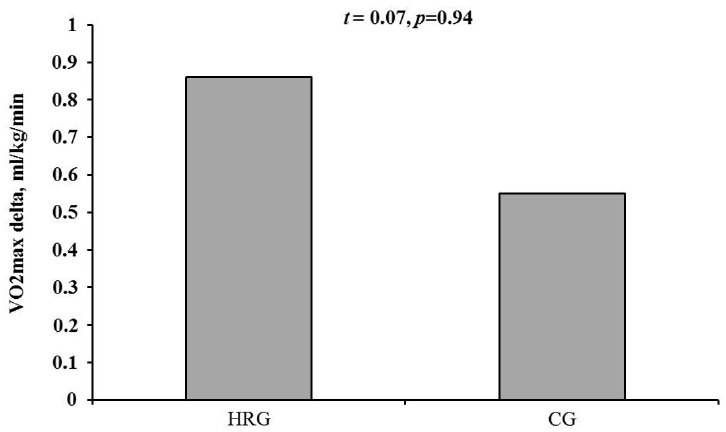
However, the standing long jump results followed by hippotherapy did not show a significant difference between the two groups, although there was an increased tendency in the HRG (12.60 ± 24.49 cm) compared to the CG (2.73 ± 36.62 cm) (p<0.05) (See Fig. 2-A). Standing long jump results were tested using a delta t value (pre and post) to correct type II error according to the baseline difference between two groups. Knee extension 60° results showed an increased tendency in the HRG (37.75 ± 40.79 Nm) compared to the CG (12.53 ± 42.85 Nm). However, there was no significant difference between the two groups (p<0.05) (See Fig. 2-B). Cardiorespiratory fitness results after hippotherapy showed an increased tendency in the HRG (0.86 ± 14.65 ml/kg/min) compared to the CG (0.55 ± 8.02 ml/kg/min). Also, there was no significant difference between the two groups (p<0.05) (See Fig. 2-C).
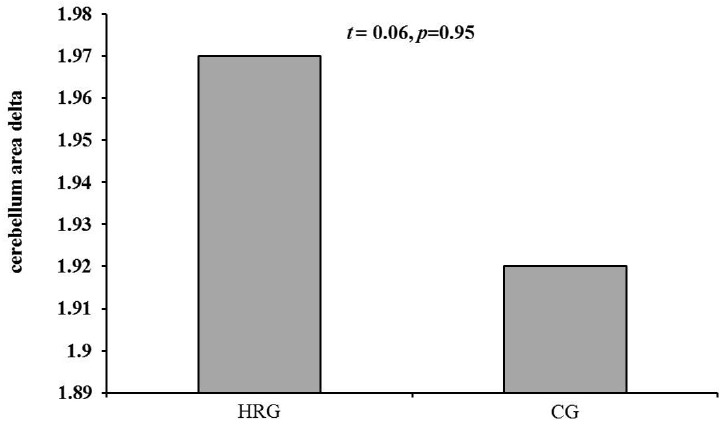
The activated insular area in the brain after hippotherapy was decreased in the HRG (-1.59 ± 0.99) compared to the CG (-1.14 ± 1.41) although there was no significant difference between the two groups (p<0.05) (See Fig. 3-A). The activated cerebellum after hippotherapy was increased in the HRG (1.97 ± 1.45) compared to the CG (1.92 ± 1.81). However, there was no significant difference between the two groups (p<0.05) (See Fig. 3-B). Changes in BDNF levels after hippotherapy showed an increased tendency in the HRG (166.29 ± 277.52 pg) compared to the CG (21.13 ± 686.33 pg). With regards to these levels there was not any significant difference between the two groups (p<0.05) (See Fig. 4).
DISCUSSION
This present study investigated physical characteristics and overall changes in brain functions in children with ADHD children after 32 weeks of hippotherapy. The hippotherapy training program included horse-riding rehabilitation, which ranged from basic training to independent horse riding that was recommended by the Rehabilitation Horse-Riding Association. After completion of the hippotherapy program, there was a significant decrease in body fat in HRG compared to the baseline. However, there was no significant changes in BDNF levels and activated brain function scanned by fMRI in the HRG.
The primary benefit of horse riding is to enhance gross motor function and positively change psychological factors [17]. Although previous studies focused on children with cerebral palsy [17-20], who differ from children with ADHD, we could expect that in this study hippotherapy might lead to positive changes in brain function. Accordingly, the results of the present study showed that there was a higher increase in BDNF levels in the HRG compared to the CG. However, there was no significant difference in the BDNF levels between the two groups. Hence, we cannot conclude that hippotherapy has a clear effect on neuroplasticity. Nevertheless, hippotherapy showed a positive tendency to improve BDNF levels in the HRG and, based on this result, we can expect that increased frequency in hippotherapy for ADHA children may have a significant positive effect on neuroplasticity. It is a known fact that the BDNF level is subordinate to blood concentration changes in the recovery phase due to intensity and frequency of exercise and platelet counts play an important role in changing BDNF levels during exercise [21,22]. Moreover, the BDNF level is an essential factor for the neuronal system and directly affects energy metabolism in the central and peripheral nervous area [23]. Therefore, this present study resulted in no significant change in BDNF levels because of low intensity and frequency of hippotherapy training, despite long term hippotherapy in children with ADHD.
The appropriate way to overcome neurophysiological problems, such as dopamine and norepinephrine decreases, in children with ADHD is to participate in regular physical activity [24,25]. In particular, participating in a long term horse-riding program similar to that used in this study is likely to have a positive effect on neurophysiological problems due to the graded nature of the physical activity. In addition, regular long term participation in physical activity may enhance physical fitness; therefore, a long term horse riding program might induce positive physical fitness level changes in the HRG. There was a higher increase in muscular fitness and cardiorespiratory fitness in the HRG compared to the CG. However, there was no significant difference between the two groups. Based on this result, we can assume that long term hippotherapy training might induce a long-lasting threshold point after the adaptation of training [26,27], which might not continuously enhance physical fitness in this study.
We expected hippotherapy to result in positive changes in BDNF levels and brain function due to the changing neuroplasticity in children with ADHD. These changes particularly pertain to the activated cellular energy metabolism and adenosine monophosphate-activated protein kinase (AMPK) enhancement that improves lipid metabolism in skeletal muscles [28,29]. Moreover, horse riding has been known to positive change motor control and is likely to decrease anterior insula area activity and increase cerebellum area activity in children with ADHD [30]. However, this present study showed that there was no significant difference in the brain function of ADHD children after hippotherapy, although there was a positive tendency in brain function. This study also expected to see positive changes in the awareness of children with ADHA according to the activated anterior insula area after hippotherapy [31]. However, it seems that big individual differences in the severity of ADHD in the study participants might cause no significant results. Further studies should consider effective sampling methods an a large enough sample based on previous studies’ statistical values to find out the clear causes of changing BDNF levels in children with ADHD.
Recent studies reported that there was a positive association between cognitive function and fitness level in children with ADHD following increased physical activity [32,33]. Therefore, this study also expected to show a significant increase in the activation of the cerebellum area and that this increase would be directly related to cognitive function. However, there was an increased tendency of activation in the cerebellum area in the HRG compared to the CG without significant increase. We assumed that these results might be the result of the low intensity and frequency of hippotherapy training. Thus, a modified hippotherapy training model for children with ADHD would be ideal in order to evoke positive changes in physical and psychological factors. Such a program would consider training volume including intensity and frequency, threshold point, and the level of training adaptation.
CONCLUSIONS
This study found that, for children with ADHD, hippotherapy (once a week for 32 weeks) significantly decreased body fat and increased BDNF levels, activated the anterior insula and cerebellum areas with no significant changes. Therefore, this study suggests that hippotherapy training should be modified and developed further to increase the efficacy of hippotherapy for children with ADHD.
Acknowledgements
This work was supported by the National Research Foundation of Korea Grant funded by the Korean Government (NRF-2013S1A5A8022825)