Moderate exercise training is more effective than resveratrol supplementation for ameliorating lipid metabolic complication in skeletal muscle of high fat diet-induced obese mice
Article information
Abstract
[Purpose]
The aim of this study was to compare the effectiveness of moderate exercise training or resveratrol supplementation with a low fat diet on lipid metabolism in the skeletal muscle of high fat diet-induced obese mice.
[Methods]
C57BL/6J mice (5 weeks old, n = 30) were fed a high fat diet (45% fat) for 8 weeks first to make them obese. Afterward, all the mice were fed a low fat diet during 8 weeks of intervention with moderate exercise training and resveratrol supplementation. Before the intervention, the mice were separated into 3 groups: low-fat diet control (HLC; n = 10), low fat diet with resveratrol (HLR; n = 10) or low fat diet with exercise (HLE n = 10). The exercise group (HLE) performed treadmill running for 30-60 min/day at 10-22 m/min, 0% grade, 5 times/week for 8 weeks, while the resveratrol group (HLR) received a daily dose of resveratrol (10 mg/kg of body weight), 5 days/week for 8 weeks.
[Results]
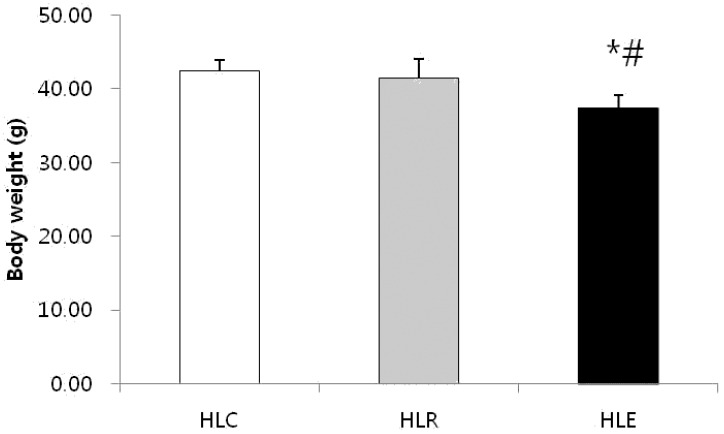
Body weight was significantly reduced in HLE. Further, the lipogenesis marker SREBP and the inflammatory cytokine TNF-α were significant reduced in HLE. However, there was no significant effect from resveratrol supplementation with a low fat diet. Taken together, exercise training with a low fat diet has the positive effect of ameliorating lipid disturbance in the skeletal muscle of high fat diet-induced obese mice.
[Conclusion]
These findings suggest that exercise training with a low fat diet is most effective to improve lipid metabolism by reducing lipogenesis and inflammation in the skeletal muscle of high fat diet-induced obese mice.
INTRODUCTION
Recently, the polyphenol resveratrol (3,5,4-trihydroxystilbene) was spotlighted as a health functional food throughout the world. Resveratrol is known to increase the expression of enzymes related to nitric oxide synthesis, reduce blood pressure and protect heart function by inhibiting platelet aggregation [1-3]. Further, previous studies reported that resveratrol delayed or protected against neuro degenerative disease and stroke induced brain damage [4,5]. Other studies showed that resveratrol prevents aging and increases the life span by activating sirtuin, which regulates gene transcription, DNA repair, cell signaling and apoptosis as its key role in promoting longevity [6,7].
Ungvari et al. [8] reported that resveratrol improves endurance capacity by increasing mitochondria number, reducing reactive oxygen species (ROS) and ameliorating lipid metabolism. This result was supported by the finding that resveratrol-mediated activation of AMPK increased intercellular NAD+ level [9,10] and decreased PGC-1α (peroxisome proliferator activated receptor-γ coactivator-1α) acetylation through deacetylation of SIRT-1 (silent mating type information regulation 2 homolog) [11,12]. In addition, resveratrol contributes to changing the type of muscle twitch fiber and PGC-1α activation by improving the function of mitochondria biogenesis [13]. Surprisingly, the maximal oxygen consumption was significantly increased and average running distance was about 2 times as long in the resveratrol supplementation group compared to the control group [13].
In obese research, it was reported that resveratrol supplementation reduced lipid volume, body weight and glucose tolerance, and improved insulin sensitivity by increasing metabolic rate [14]. In human studies, it was shown that resveratrol supplementation (150 mg/kg) in obese individuals significantly increased citrate synthase activity but not in the control group. However, there was no significant difference in mitochondria biogenesis and aerobics capacity [14].
Excessive nutrient-induced accumulated fat is known to result in chronic low inflammation, especially the inflammatory cytokines TNF-α (tumor necrosis factor-α) and interleukin-6 (IL-6), which are higher in obese individuals than in normal weight individuals [15,16]. Also, it has been reported that these inflammatory cytokines inhibit lipid oxidation in skeletal muscle, but increase lipid synthesis. Borst et al. reported that adipose tissue-induced TNF-α increased the enzyme activity related to lipid synthesis such as SREBP-1c (sterol regulatory element binding protein-c), ACC (acetyl-CoA carboxylase) and FAS (fatty acid synthase) [17].
It is well known that moderate endurance exercise training reduces body weight and glucose tolerance while improving insulin sensitivity, maximal oxygen consumption and mitochondrial biogenesis [18-20]. These positive results from exercise training were similar with the results of previous studies on resveratrol supplementation [8-13]. Thus, resveratrol was believed to have efficacy as an exercise mimicker [18,21,22].
On the other hand, previous studies on diet intervention for obese-induced metabolic complications reported that low fat and nutrient balanced diets improved pro-inflammatory milieu through the reduction of CRP, IL-6, IL-18, TNF-α, insulin sensitivity, endothelial function, metabolic syndrome, Type 2 DM and morbidity of CHD (coronary heart disease) [23,24].
Similarly, Calder et al. showed that a low fat diet was a key factor for positive changes in weight loss, lipid metabolism and inflammatory cytokine response [25]. Further, it was suggested that exercise intervention was more effective than low fat diet treatment for reducing hepatic steatosis, but both exercise and a low fat diet were effective in improving the anti-inflammatory response and metabolic outcomes [26,27].
Nonetheless, there are few studies comparing the effects of exercise training and resveratrol supplementation. In addition, there is little research to our knowledge, comparing the effects of exercise training and resveratrol supplementation using a low fat diet as a base in high fat diet-induced obese mice. The effect of a low fat diet has been well documented and a low fat diet can be easily applied as a treatment for obese-induced metabolic complications. Also, in previous rodent studies, there were positive effects observed with resveratrol dosage (25-30 mg/kg/day or 400 mg/kg/day). These doses for mice would be equivalent to 2,100 or 28,000 mg/kg/day for humans (70 kg). Thus, these doses are unlikely to be obtained from natural or functional foods [9,13].
We hypothesized that if a low fat diet were combined with either exercise training or resveratrol supplementation, the efficacy of resveratrol as an exercise mimicker would be clearly identified. Consequently, we investigated the effect of either moderate exercise training or resveratrol supplementation with a low fat diet on lipid metabolism in the skeletal muscle of high fat diet-induced obese mice.
METHODS
Animals care and diet
Male C57BL/6 mice (5-weeks-old) were purchased from Raonbio (Seoul, Korea) and housed in standard cages placed in a room at 22 ± 2.0℃, 55 ± 10% relative humidity, and a 12 hour-light/12 hour-dark cycle. All mice consumed a commercial diet and tap water ad libitum for 1 week. Mice were randomly assigned to three groups: (1) HFD for 8 weeks and low-fat diet for 8 weeks as the control (HLC; n = 10); (2) HFD for 8 weeks and low-fat diet for 8 weeks with resveratrol (HLR; n = 10); (3) HFD for 8 weeks and low-fat diet for 8 weeks with exercise (HLE; n = 10). The mice were weighed every week during the experimental period. Commercially available dried resveratrol (Sigma Aldrich, St Louis, MO) was used, dissolved in dimethyl sulfoxide. The control (HLC) and exercise (HLE) groups were orally administered with 100 μl of dimethyl sulfoxide as a vehicle. The resveratrol group was orally gavaged with resveratrol (10 mg/kg body weight) dissolved in dimethyl sulfoxide. This dose is similar to the amount that humans consume from natural or functional foods. Each treatment was administered once per day, 5days/week for 8 weeks [27,28].
Exercise protocol
Exercise training was performed on a motor treadmill at moderate intensity for 8 weeks, 5 days/week for 30-60 min/day during the dark cycle. 1 week of adaptation was employed for treadmill running (with the speed of 8 m/min). Warm-up for all exercises was conducted for 5~10 minutes at the speed of 8-10 m/min and the main exercise was performed at the speed of 10-22 m/min for 30~60 minutes, following the principle of cumulative overload.
This exercise intensity corresponds to 65-70% of maximal oxygen uptake [29]. To control for any stress associated with the training protocol, animals in the control and resveratrol groups were exposed to the same noise and handling as the exercise groups.
Tissue preparation
The animals were sacrificed 24 hours after the 8th week of exercise training. Skeletal muscle (gastrocnemius muscle) tissue was stored at -70℃ until analysis. This study used the gastrocnemius muscle because it was determined that aerobic exercise for 8 weeks can affect the muscles.
RNA extraction and quantitative RT-PCR
Total RNA was isolated from the skeletal muscle tissue of each mouse using Trizol (Invitrogen, Carlsbad, CA, USA). Quantitative real-time RT-PCR was conducted using the Biorad CFX96(Biorad, USA) PCR system. The mRNA levels were normalized to the β-actin mRNA levels.
Statistical methods
The data was analyzed using SPSS ver. 20.0 (SPSS, Inc., Chicago, IL, USA) to obtain means ± standard deviations. To investigate the effects of moderate exercise and resveratrol intake on body weight, inflammation, and lipid metabolism in skeletal muscle, all the groups were compared by a one-way ANOVA. The differences were considered statistically significant at α =0.05.
RESULTS
Changes of body weight
Fig. 1 shows body weight in the low fat diet, resveratrol supplementation and moderate exercise training groups following consumption of a low fat diet for 8 weeks after 8 weeks of a high fat diet was used to induce obesity in mice. The body weight of HLE was significantly reduced compared to HLC and HLR (p<0.05, Fig. 1).
Inflammatory marker mRNA expression
Fig. 2. shows mRNA expression of inflammatory cytokines in low fat diet, resveratrol supplementation and moderate exercise training groups following consumption of a low fat diet for 8 weeks after 8 weeks of a high fat diet was used to induce obesity in mice.

Inflammatory cytokines mRNA expression in low fat diet, resveratrol supplementation and moderate exercise training groups following consumption of a low fat diet for 8 weeks after 8 weeks of a high fat diet was used to induce obesity in mice. Values represent means ± SD.
* P < .05 versus HLC, # P < .05 versus HLR.
The TNF-α mRNA expression of HLE was significantly reduced compared to HLC and HLR (p<0.05, Fig. 2).
Lipolysis mRNA expression
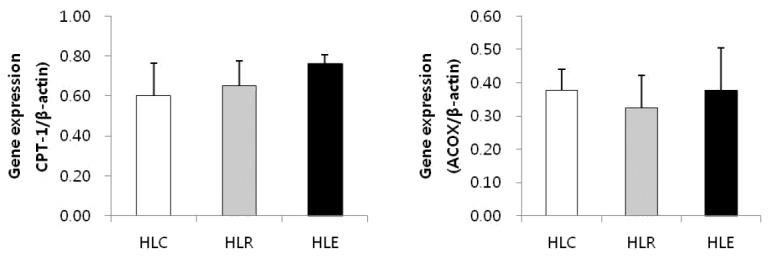
Fig. 3 shows lipolysis markers mRNA expression in low fat diet, resveratrol supplementation and moderate exercise training groups following consumption of a low fat diet for 8 weeks after 8 weeks of a high fat was used to induce obesity in mice.
Lipogenesis mRNA expression
Fig. 4 shows the mRNA expression of lipogenesis markers in low fat diet, resveratrol supplementation and moderate exercise training groups following consumption of a low fat diet for 8 weeks after 8 weeks of a high fat diet was used to induce obesity in mice.
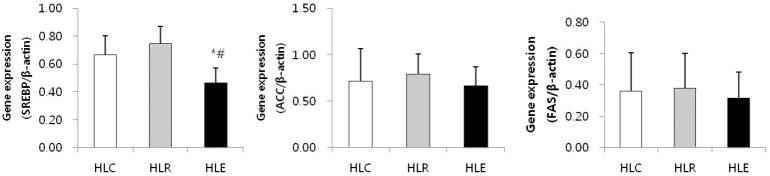
mRNA expression of lipogenesis markers in low fat diet, resveratrol supplementation and moderate exercise training groups following consumption of a low fat diet for 8 weeks after 8 weeks of a high fat diet was used to induce obesity in mice. Values represent means ± SD.
* P < .05 versus HLC, # P < .05 versus HLR.
SREBP mRNA expression of HLE was significantly reduced compared to HLC and HLR (p<0.05, Fig. 4).
DISCUSSION
Since calorie restriction is known to increase life span, the popularity of one meal per day, low calorie consumption and interval fasting diets has increased.
A previous research reported that the effects of calorie restriction are caused by a protein called sirtuin [30]. Sirtuin is activated by NAD+-dependent deacetylases during the fasting condition in the brain, liver and kidney [31]. Further, it has been shown that sirtuin regulates metabolism and gene expression by stimulating the activation of SIRT-1 deacetylation. It even recovers damaged genes by activating PGC-1α, inhibiting weight gain and aging [6,11,13]. These results were supported by studies reporting a reduction in body weight changes and increased life span in the artificially sirtuin-activated group compared to the control group. However, after treatment with high carbohydrate or high fat diets, the positive effects of sirtuin vanished [6,30,31].
It seems that the reason why resveratrol research has been spotlighted may be related to the increment of SIRT-1. Interest in resveratrol supplementation increased because the effects coincided with those of calorie restriction [6]. Also, research interest in resveratrol was dramatically increased after a few studies reported that resveratrol supplementation could mimic the effects of exercise such as body weight reduction, mitochondria biogenesis improvement, endurance performance enhancement and energy consumption increment [5,7].
Body weight in this study was significantly reduced by moderate exercise training but not by resveratrol supplementation. This result contrasted with a previous study that reported significant weight reduction following resveratrol supplementation (200-400 mg/kg/day) in obese mice [13]. However, our previous results also showed no significant effect on body weight. In this research, 10 mg/kg each of quercetin and resveratrol were injected into high fat diet-induced obese mice for 8 weeks. The body weight of the group was then compared to the moderate exercise training group. Body weight was significantly reduced only in the exercise group, which coincides with other studies [28,32,33]. Further, it seems that the dose of resveratrol was not enough to reduce body weight.
It seems that even if the effect of polyphenols such as quercetin and resveratrol depends on the amount used, intervention with moderate exercise training is more effective in reducing body weight.
Adipocyte-produced inflammatory cytokines such as IL-6 and TNF-α have been reported to inhibit lipid oxidation but to increase lipid synthesis in skeletal muscles. Especially, TNF-α activates SREBP-1c, ACC and FAS, which are related to lipid synthesis. However, ACOX and CPT-1 were inhibited, which deteriorated insulin sensitivity [17,18].
In this research, TNF-α gene expression was significantly decreased in HLE compared to HLC and HLR, but there was no significant difference in IL-6 and MCP-1 gene expression. These results show that resveratrol supplementation had no positive effects on inflammatory cytokine production compared to moderate exercise training. Further, CPT-1 and ACOX were not significantly different but were slightly increased only in the HLE group. There was no change in the HLR group. The lipogenesis markers ACC, FAS and SREBP were slightly decreased in HLE, but only SREBP was significantly lower in HLE. In contrast, there was no significant difference in HLR. This result shows that moderate exercise training might inhibit lipid synthesis and promote lipid oxidation in the skeletal muscle of high fat diet-induced obese mice.
In addition, in the review of our previous research that compared the effects of moderate exercise training and quercetin supplementation for 8 weeks in 12-week high fat diet-induced obese mice, SREBP, FAS and ACC were significantly decreased in the exercise group compared to the polyphenol supplementation group. At the same time, LEPTIN and ACOX in exercise group were positively changed [33].
In a similar experiment, we analyzed the effects of either resveratrol supplementation or moderate exercise training on inflammation cytokines, mitochondria biogenesis, lipolysis and lipogenesis markers in the soleus and tibialis anterior muscles after exhaustive exercise in high fat diet-induced obese mice. We found that IL-6, TNF-α and MCP-1 were significantly reduced and so were MCD, ACC and SREBP-1 [32].
In previous studies of obese animals, polyphenol compounds such as resveratrol and quercetin appeared to mimic the effects of calorie restriction or exercise training. However, the dosage, duration and side effects of polyphenols should be studied further [5]. The effects of resveratrol with a low fat diet did not exceed the effects of only a low fat diet in this study. On the other hand, the effects of moderate exercise training with a low fat diet were much higher compared to either a low fat diet alone or resveratrol with a low fat diet in terms of improving lipid metabolism in the skeletal muscles of high fat diet-induced obese mice. This research evaluated only the resveratrol dose (10 mg/kg) similar to the amount that humans can obtain from natural or functional foods. The effect of resveratrol supplementation was not enough to reduce body weight, whereas moderate exercise training had a positive effect.
In conclusion, moderate exercise training combined with a low fat diet was more effective than resveratrol supplementation with a low fat diet in ameliorating lipid metabolic complications in the skeletal muscles of high fat diet-induced obese mice.
Acknowledgements
This work was supported by the Chungnam National University (CNU-2014)
All experiments were approved by the Animal Care and Use Committee at the Chungnam National University (CNU-00202).