The effects of either resveratrol or exercise on macrophage infiltration and switching from M1 to M2 in high fat diet mice
Article information
Abstract
[Purpose]
The aim of this study was to compare the effectiveness of either resveratrol supplementation or exercise training on macrophage infiltration and switching from M1 to M2 kupffer cells in high fat diet mice.
[Methods]
C57BL/6 mice were separated into 5 groups: normal diet (ND; n = 6), high-fat diet (HD; n = 6), high-fat diet with resveratrol (HR; n = 6), high-fat diet with exercise (HE; n = 6) or high-fat diet with resveratrol and exercise (HRE; n = 6). Resveratrol supplementation mice were orally gavaged with resveratrol (25mg/kg of body weight) dissolved in 50% propylene glycol. Exercise mice ran on a treadmill at 12-20 m/min for 30-60 min/day, 5 times/week for 12 weeks.
[Results]
After 12 weeks of intervention, the liver was analyzed. F4/80 expression was evaluated by western blot while CD11c and CD163 mRNA expressions were evaluated by RT-PCR. The weights of the body and liver were significantly increased in the HD and HR group compared to the ND group (p < 0.01). However, the weights were most effectively reduced in the HE and HRE groups compared to the HD group (p < 0.05). The macrophage marker, F4/80 expression was significantly lower in the HE and HRE groups compared to the HD group (p < 0.05). mRNA expression of the M1 macrophage marker, CD11c, in the HD group was significantly increased compared to the ND group (p < 0.01). mRNA expression of the M2 macrophage specific marker, CD163, in the HE and HRE groups were significantly increased compared to the HD group (p < 0.05). The mRNA expressions of TLR4, ICAM-1 and VCAM-1, which induce pro-inflammatory cytokine production, were strongly decreased in the HR, HE, and HRE groups compared to the HD group.
[Conclusion]
These results suggest that moderate exercise training inhibits macrophage infiltration and up regulation of CD163 expression. However, resveratrol supplementation is not enough to ameliorate obesity-induced macrophage infiltration and switching.
INTRODUCTION
The liver has major functions in the coordination of metabolism. Hepatocytes are involved in lipid metabolism and the release of inflammatory cytokines [1]. In obesity, inflammatory cytokines released from adipose tissue move outside the liver through the portal vein and can directly interfere with liver functions. Especially, Kupffer cells are important sources of inflammatory cytokines within the liver and are the inhabitant macrophages in the liver. During steatosis, the recruitment of macrophages into the liver can alter cell distribution, thereby also changing Kupffer cell morphology and function [2]. Macrophages have a highly plastic phenotype that allows them to specialize and display polarized functional properties, such as inflammatory or anti-inflammatory actions in response to cytokine products [3].
The so-called classical activated or “M1” macrophages secrete high amounts of pro-inflammatory mediators while the alternatively activated “M2” macrophages are low pro-inflammatory cytokine producers. In obesity, the balance between M1 and M2 macrophages is disturbed. The M1 macrophage specific marker, CD11c, is increased in high fat diet conditions, whereas the M2 macrophage specific marker, CD163, is decreased [4].
Previous reports showed that the expression of adhesion molecules in adipose tissue was increased in the obese condition [5,6] and a different study indicated that HFD induced obese mice exhibited higher expression of Intercellular adhesion molecule 1(ICAM-1) and Vascular cell adhesion molecule 1(VCAM-1) in adipose tissue. On the other hand, obese mice did not exhibit macrophage infiltration into adipose tissue after ICAM-1 antagonist treatment [7]. Thus, adhesion molecules affect obesity-induced macrophage infiltration into adipose tissue.
Regular exercise can prevent chronic inflammation [8,9] and reduce the state of fibrosis as a marker of hepatic injury [10,11]. A previous study showed that exercise training reduces inflammatory cytokines in adipose tissue by suppressing several adipocyte-differentiation genes [12]. Cellular molecule recognition by toll-like receptors such as Toll-like receptor 4 (TLR4) induces inflammatory cytokine production [13]. A review [14] on the topic demonstrated that TLR4 expression in exercise groups are lower than in control groups. One possible explanation is that exercise training suppresses inflammatory cytokines in obese mice, which might be controlled by the decrease in TLR4.
Resveratrol has been shown to possess anti-inflammatory and antioxidant properties [15,16]. Resveratrol also attenuates hepatic lipid metabolism [17]. A recent study demonstrated that resveratrol exerts inhibitory effects on adipocyte differentiation, which accompanies the down-regulation of several adipocyte-specific genes [18].
Although resveratrol or exercise has been suggested to reduce hepatic fat accumulation, the beneficial effect on macrophage infiltration is unclear. Additionally, the protective effect of high-fat induced polarization from M2 to M1 is unknown.
Therefore, the purpose of this study was to compare the effectiveness of either resveratrol supplementation or aerobic exercise training on macrophage infiltration, shifting from M1 to M2 and inflammation induced genes in high fat diet mice.
METHODS
Animals and diet
4-week-old male C57BL/6 (Central Experiment Animal, Korea, n = 30) mice were housed in cages (5 mice per cage) in a standard experimental laboratory, at a temperature of 22 ±2℃, with 60 ± 5% humidity. After a one-week acclimatization period, the mice were fed either a high fat diet (45% of energy from fat, Orient Bio Inc., # D12451) or a normal diet (10% of energy from fat, Orient Bio Inc., # D12451) ad libitum for 12 weeks.
The mice were divided into 5 groups: normal diet (ND; n = 5), high fat diet (HD; n = 5), high fat diet with resveratrol (HR; n = 5), high fat diet with exercise (HE; n = 5), high fat diet with resveratrol plus exercise (HRE; n = 6). The exercise groups performed treadmill running for 23-60 min/day at 10-22 m/min, 5 times/week for 12 weeks. Food consumption and body weight were monitored daily and once per week, respectively, using a standard table scale. At the end of the experimental period, liver tissues were dissected, weighed and immediately frozen.
The protocols were approved by the Animal Experimentation Committee of Chungnam National University (CNU-00202), Korea.
Exercise protocol and resveratrol supplement
In the early phase of moderate exercise, all the mice in the exercise group ran at a speed of 8-10 m/min for 10 minutes on an automatically operated rodent treadmill. Afterward, the exercise speed and time were gradually increased up to 10-22 m/min for 30-60 minutes.
The volume of the exercise training protocol represents 60-75% of the maximum oxygen consumption of the rodents for a moderate intensity exercise program [19]. A foam sponge was placed in the back of each treadmill lane to prevent injury to the animal. Electrical shock was not used during the treadmill run. While the mice in the exercise group were training, the control mice were exposed to environmental stress as well as vibration and noise from the treadmill, and their food and water supply were removed. Resveratrol supplemented mice were orally gavaged with resveratrol (25mg/kg of body weight) dissolved in 50% propylene glycol [20]. The other group received only the vehicle. Each treatment was administered once a day, 5 times/week for 12 weeks before tissue collection.
Western blot
Forty micrograms of protein was separated on 7.5-10% SDS/PAGE and transferred onto nitrocellulose membranes. After blocking for 1 h in 5% skim milk solution, the membranes were incubated with specific antibodies to F4/80 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), and β-actin (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Membranes were then exposed to an anti-rabbit secondary antibody conjugated to horseradish peroxidase (Santa Cruz Biotechnology, Santa Cruz, CA, USA) for 1 h at room temperature. The signals were detected by chemiluminescence using the ECL detection reagent (GE Healthcare, Piscataway, NJ, USA). The bands were scanned with a ChemiDoc XRS System (Bio-Rad Laboratories) with a cooled 12-bit camera and quantified by densitometry. Levels of target proteins were normalized to β-actin values.
RNA extraction and RT-PCR
Total RNA was isolated from 20 mg of liver tissue using Trizol (Qiagen, Hilden, Germany), according to the manufacturer's instructions. RNA was quantified by spectroscopy (NanoDrop, Wilmington, DE). From each sample, 1 μg of total RNA was reverse transcribed to cDNA with primers of random and oligo(dt) using a high-capacity RNA-to-cDNA kit (Roche, Mannheim, Germany) in a total volume of 20 μL, as described in the high-capacity RNA-to-cDNA kit protocol. The amplification was performed in a total volume of 20 μL, which included 2 μL of cDNA, 1 μL of each primer (10 pmol/ μL), and 16 μL of DEPC (Diethyl pyrocarbonate) water. Reactions were carried out in an ABI 7500 system using the following thermal cycling parameters: 95℃ for 2 min, and then 38-40 cycles of 95℃ for 30sec, the appropriate annealing temperature for 30sec, and 72℃ for 2 min. All samples were examined in parallel for β-actin. All primer sequences were designed using IDT (Skokie, IL), verified using the National Center for Biotechnology Information (NCBI) BLAST feature, and purchased from MWG Biotech (Huntsville, AL).
Statistical analysis
Data on the body and liver weight are presented as mean ± SD. Western blotting and RT-PCR data were analyzed using one-way analysis of variance (ANOVA) with LSD post-hoc tests. Statistical significance was defined as P <0.05. Statistical analysis of the data was performed using SPSS statistical software (Version 18.0).
RESULTS
The change in body and liver weight
Fig. 1 shows the body and liver weights of high-fat diet fed mice.
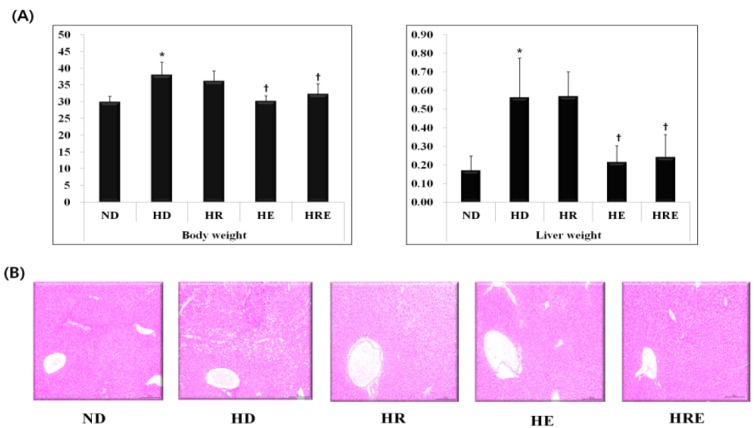
The change in body and liver weights, and lipid droplets in liver of high fat diet mice. All values are expressed as means±SD. P-values are the result of post hoc LSD test when the one-way ANOVA was significant. ND: normal diet; HD: high fat diet; HR: high fat diet with resveratrol; HE: high fat diet with exercise; HRE: high fat diet with resveratrol+exercise. * p < .05 different from the ND group, +p < .05 different from the HD group.
The body and liver weights were significantly increased in the HD and HR groups compared to the ND group (p <0.01). However, the weights were most effectively decreased in the HE and HRE group compared to the HD group (p <0.05). H&E staining showed some lipid droplets in the liver (Fig. 1B) and the HD groups had more lipid droplets than the ND group.
F4/80 changes in the liver
Fig. 2 shows the expression of the macrophage marker, F4/80, in the high-fat diet fed mice.
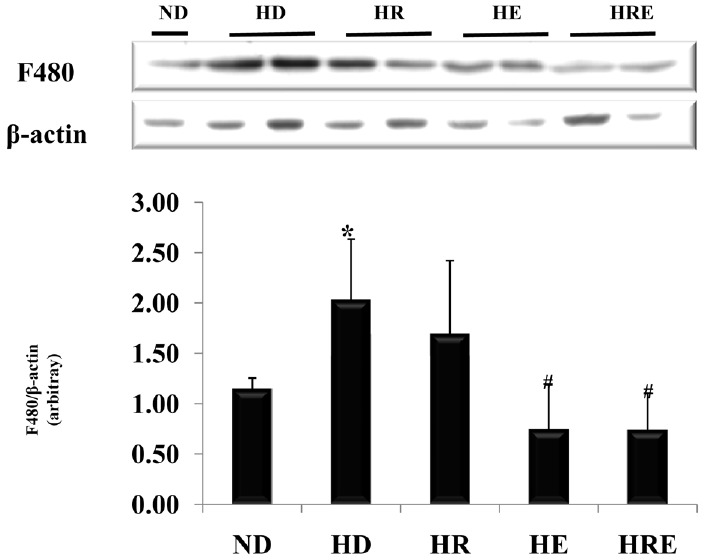
Expression of the macrophage marker, F480, in liver of high fat diet mice. All values are expressed as means±SD. P-values are the result of post hoc LSD test when the one-way ANOVA was significant. ND: normal diet; HD: high fat diet; HR: high fat diet with resveratrol; HE: high fat diet with exercise; HRE: high fat diet with resveratrol+exercise. * p < .05 different from the ND group, +p < .05 different from the HD group.
The F4/80 expression was significantly increased in the HD group compared to the ND group (p < 0.05). Nevertheless, F4/80 expression was significantly decreased in the HE and HRE groups compared to the HD group (p < 0.05). However, the HR group was not significantly affected compared to the HD group.
CD11c and CD163 changes in the liver
Fig. 3 shows the mRNA expressions of CD11c and CD163. mRNA expression of the M1 macrophage marker, CD11c, was markedly increased in the HD group compared to the ND group (P < 0.01), but the CD11c mRNA expression was not significantly different in the HE, HR, and HRE groups.

mRNA expression of the M1 macrophage marker CD11c and the M2 macrophage marker CD163 in liver of high fat diet obese mice. All values are expressed as means±SD. P-values are the result of post hoc LSD test when the one-way ANOVA was significant. ND: normal diet; HD: high fat diet; HR: high fat diet with resveratrol; HE: high fat diet with exercise; HRE: high fat diet with resveratrol+exercise. * p < .05 different from the ND group, +p < .05 different from the HD group.
mRNA expression of the M2 macrophage specific marker, CD163, was significantly decreased in the HD group compared to the ND group (p < 0.05). However, CD163 expression was most significantly increased in the HE and HRE groups compared to the HD group (p < 0.05).
TLR4, ICAM-1, and VCAM-1 changes in the liver
Fig. 4 shows the mRNA expressions of TLR4, ICAM-1, and VCAM-1

mRNA expression of TLR4, ICAM-1, and VCAM-1 in liver of high fat diet mice. All values are expressed as means±SD. P-values are the result of post hoc LSD test when the one-way ANOVA was significant. ND: normal diet; HD: high fat diet; HR: high fat diet with resveratrol; HE: high fat diet with exercise; HRE: high fat diet with resveratrol + exercise. * p < .05 different from the ND group, +p < .05 different from the HD group.
The mRNA expression of TLR4 was not significantly different in the ND and HD groups. However, TLR4 mRNA expression was significantly decreased in the HE, HR, and HRE groups compared to the HD group (p <0.05).
mRNA expression of the adhesion molecules ICAM-1 and VCAM-1 were significantly increased in the HD group compared to the ND group (p < 0.05). Further, ICAM-1 and VCAM-1 mRNA expression were most effectively decreased in the HE, HR, and HRE groups compared to the HD group (p <0.05).
DISCUSSION
A high fat diet is known to increase body weight and fat mass and obesity is recognized as a social health problem. Moderate exercise increases energy expenditure and has been recommended for the treatment of obesity [21]. Also, it is known that resveratrol treatments reduce body weight and liver weight compared to non-treatment in animals [22]. Thus, it was meaningful to compare the effectiveness of exercise and resveratrol on obesity-induced metabolic complications.
In our results, body and liver weights were markedly increased in the HD and HR groups compared to the ND group (Fig. 1). Nevertheless, the exercise trained mice (HE and HRE) had a lower body and liver weight than the high fat diet fed (HD) mice (p < 0.05). In support of our results, a previous study demonstrated that exercise has positive effects on the reduction of body weight and fat mass [23]. Another study showed that regular exercise reduced liver mass, indicating that reduced liver mass might lead to reduced hepatic triglyceride accumulation [24]. Therefore, our results suggest that exercise training effectively regulated high fat diet-induced liver and body weight and reduced hepatic triglyceride accumulation. The previous study reported that a high-fat diet with resveratrol supplementation reduced fat content and body weight gain [25]. In contrast, this effect was not observed in other studies [26,27]. In our experiments, there was no significant change in the HR group compared to the HD group. It seems that the effect of resveratrol depends on the dose and the length of supplementation.
Especially, obese-induced macrophage infiltration is a major source of pro-inflammatory cytokine expression [24]. Previous studies have noted that the high levels of pro-inflammatory cytokine production after high-fat-diet treatment were decreased by the inhibition of macrophage infiltration in obese animals [28,29]. However, the effect of exercise or resveratrol with respect to the inhibition of macrophage infiltration in the liver is still not established.
The present study found that expression of the macrophage marker, F4/80, was significantly increased in the HD group compared to the ND group. However, the expression of F4/80 was significantly lower in the exercise groups than in the high fat diet group. The present finding that exercise training reduces the expression of adipose tissue F4/80 mRNA coincides with previous studies [13,29].
In this study, resveratrol supplementation was not enough to reduce F4/80 in the liver. The reason for this has not been fully explained. However, Bujanda et al., [30] reported that resveratrol inhibited lipid peroxidation and reduced inflammation while simultaneously increasing antioxidant enzymes in obese rats. Another study reported that over 20 weeks of dietary intake of polyphenol alleviated hepatic fat accumulation in obese mice [31]. Therefore, more research is needed to elucidate the in vitro and in vivo long term effects of resveratrol treatment at various doses in obese rodents. To summarize, 12 weeks of exercise training had a more positive effect than resveratrol treatment in ameliorating high fat diet induced macrophage infiltration.
M1 and M2 macrophage activation represent two ends of the macrophage polarization state [24], which may accelerate or decelerate metabolic disease. In this study, mRNA expression of the M1 macrophage marker, CD11c, markedly increased in the HD group compared to the ND group (P <0.01).
Previous research reported that exercise training may inhibit M1 macrophage infiltration into visceral adipose tissue [24]. Treatment with the CD11c antagonist attenuated inflammation via the inhibition of M1 macrophage infiltration in obese mice [32]. Nevertheless, the results of this study showed that there was no difference in CD11c mRNA expression between the exercise and resveratrol treatment groups in the liver. In contrast, mRNA expression of the M2 macrophage specific marker, CD163, was reduced in the HD group compared to the ND group (p < 0.05). In the HE and HRE groups, CD163 mRNA expression was significantly increased compared to the HD group (p < 0.05). Macrophage activation has been categorized into two separate polarization conditions known as M1 and M2 [33]. M1 macrophages powerfully express TLR4, which produces pro-inflammatory cytokines such as TNF-a. In contrast, M2 macrophages generate IL-10, which suppresses pro-inflammation states. In a previous study, obese animals exhibited a phenotypic switch from M2 macrophages to M1 macrophages in adipose tissue [34]. Another study demonstrated that exercise training reduced the expression of inflammatory cytokines and M1 macrophage markers in the adipose tissue of obese animals [24]. It is possible that exercise training suppressed macrophage infiltration in the liver by upregulating CD163 in obese mice.
In the present results, mRNA expression of TLR4, which induces pro-inflammatory cytokine production, were strongly decreased in HR, HE, and HRE compared to HD mice. Moderate exercise induces glucocorticoid secretion, which inhibits the expression of TLR2 and TLR4 [35]. The reduced level of TLR4 in the exercise trained group may have been caused by the stimulation of glucocorticoids.
Also, this study found that the HD group had higher mRNA expressions of ICAM-1 and VCAM-1 in the liver. Previous studies reported that the expression of adhesion molecules in visceral adipose tissue was increased by obesity [36,37]. In another study, obese mice did not exhibit macrophage infiltration into adipose tissue after ICAM-1 antagonist treatment [38]. A different study showed that plasma ICAM-1 concentrations were markedly decreased by moderate exercise training in metabolic disease patients without concomitant weight loss [39]. More research is required to further elucidate the effects of exercise or resveratrol on the weakening of adhesion molecules in the liver caused by the anti-inflammatory effects of exercise and resveratrol.
Exercise and resveratrol are currently believed to induce crucial events leading to the reduction of inflammatory cytokines. However, it is still unclear how exercise or resveratrol down-regulates the inflammatory state. The present study shows that the body and liver weights of the HD group were significantly increased compared to the ND group. However, the body and liver weights of the exercise groups were significantly decreased after long-term aerobics training, compared to the high fat diet group. In addition, it was identified that moderate exercise training controlled the downregulation of F4/80 and the upregulation of CD163. Also, the HE, HR, and HRE groups exhibited lower expression of TLR4, ICAM-1, and VCAM-1 compared to the HD group.
In conclusion, it seems that moderate exercise training might contribute to the inhibition of macrophage infiltration and the shifting of M1 to M2 macrophages by suppressing TLR 4 expression and inflammation in the liver. However, resveratrol supplementation alone is not enough to ameliorate high fat diet-induced obese complications.
Acknowledgements
This work was supported by the National Research Foundation of Korea Grant funded by the Korean Government (NRF-2014S1A5A2A01016657)
All experiments were approved by the Animal Care and Use Committee at the Chungnam National University (CNU-00202).