Effects of voluntary exercise on apoptosis and cortisol after chronic restraint stress in mice
Article information
Abstract
[Purpose]
To determine whether voluntary exercise (wheel running) has the potential of relieving stress.
[Methods]
In this study, restraint stress with or without voluntary wheel running was performed for mice housed in individual cages. A total of 21 ICR male mice were assigned into control (CON), restraint stress with voluntary exercise (RSVE), or restraint stress (RS) without voluntary exercise groups (n = 7 each).
[Results]
No significant difference in body weight increase was found among the three groups, although CON and RS groups had a tendency of having smaller body weight increase compared to the RSVE group. No significant difference in the expression level of liver heat shock protein 70, Bcl-2, or p53 was found among the three groups. However, caspase-3 protein level in RS group was significantly higher than that in the other two groups. Blood cortisol concentration in RS was higher (p < 0.05) than that in RSVE or CON group. It was the lowest (p < 0.05) in the RSVE group.
[Conclusion]
Our findings suggest that apoptosis caused by chronic restraint stress might be suppressed by voluntary exercise in mice.
INTRODUCTION
It has been reported that repeated and stressful situation can cause chronic stress in animals and lead to hypothalamus-pituitary-adrenal axis change1. In addition, short-term stress can be used as a starting point to determine various hormonal changes in animals2. Restraint stress can reduce serum levels of Bcl-2, estradiol, and IGF-I but increase the levels of cortisol and progesterone3. From another perspective, carbohydrates and saturated fatty acids have been reported to be preferred by people with chronic stress, resulting in weight gain4. It has been reported that the concentration of cortisol in Cynomolgus monkey is increased by restraint stress5. On the other hand, exercise can enhance resistance to stress and suppressed anxiety. It can also reduce depression in human and animals6. After prolonged repetitive restraint stress, female rats have shown higher concentration of ovarian hormone after treadmill running exercise training7. In addition, voluntary exercise of the extremities in stroke patients can assist in the recovery of damaged limbs and their function8. Restraint stress is greater than the stress caused by forced swimming exercise2. Heat shock protein 72 (HSP 70), a representative marker of stress protein, can be increased by exercise9. Exercise can also increases the expression levels of superoxide dismutase of Mn isoform (Mn-SOD), Bax, and caspase-3 protein that inhibit apoptosis in aged rat heart10. In addition, 60 min of exercise training can increase the level of Bcl-2 while decreasing the levels of caspases 9, 3, 8, and 1211. However, high-intensity exercise can induce cell death and leukocytic apoptosis12,13.
Chronic stress can suppress elements of the hypothalamus-pituitary-adrenal cortex axis and the biosynthesis of melanin in the skin 14. Although the activity of hypothalamus-pituitary-thyroid axis is suppressed by restraint stress, light stress and dietary restrictions are not related to voluntary exercise15. Hippocampus neurons are noticeably increased by voluntary exercise, which could prevent damage to memory associated with chronic stress16. In addition, inhibition of antibacterial activities and gut function induced by chronic restraint stress can be suppressed by light activities17.
However, forced exercise after restraint stress does not have beneficial effect for impaired memory in rats18. Similarly, involuntary exercise can increase the anxiety levels and decrease the levels of vascular endothelial growth factor and brain derived neurotropic factor, both of which function in neuro-biosynthesis in the hippocampus19. On the other hand, moderate-intensity treadmill exercise or voluntary exercise can inhibit apoptosis20. It has been reported that apoptosis is suppressed and short-term memory impairment is inhibited in diabetic rats by treadmill exercise for 4 weeks after birth21. Cortisol concentration is also increased with both forced swim exercise and restraint stress2. Similarly, voluntary exercise, not forced exercise, after withdrawal symptoms of drug addiction can relieve stress22. The most common method used to train mouse is treadmill running with electrical shock. However, the electrical shock increases stress hormones and cytokines after the exercise23. Compared to forced exercise, voluntary exercise appears to provide more reliable results. Therefore, we examined the changes of apoptosis-related protein expression level and cortisol concentration with voluntary exercise after restraint stress in this study.
METHODS
Subjects
In this study, 21 male ICR (Institute of Cancer Research) mice at 5 weeks of age were used as subjects. Mice were housed in individual cages with temperature of 23-25℃, relative humidity of around 60%, and a 12-hour interval of light-dark cycle. They were assigned to the following three groups: control (CON), restraint Stress (RES), and restraint stress + voluntary wheel running (RSVE) (n = 7 per group).
Dietary composition and food intake
Commercial feed (formula-m07, Cheil Feed, Korea) was used as diet. Tab water and diet were provided to mice ad libitum.
Restraint stress
To induce stress, restraint stress is a better method than forced swim exercise2. As shown in Figure 1, mice were immobilized for 30 min/d for four weeks24.
Voluntary wheel exercise
Voluntary exercise was started when they were 6 weeks old. They ran in a wheel with diameter of 370 mm voluntarily for 4 weeks. They could enter the wheel and go back to their cages freely through a hole between the wheel and cage. In addition, the amount of rotation was counted automatically with a sensor.
Sampling
After 12 hours of fasting, rats were anesthetized with ether. The abdomen was cut open to collect blood samples from the abdominal aorta to analyze blood cortisol concentration. The collected blood samples were centrifuged (1580MER, Gyrozen, Korea) at 700 × g for 10 min. Supernatants were collected and stored in a freezer (NF-400SF, HFC, Japan) until analysis. The liver was removed and fresh frozen in liquid nitrogen to terminate its activation. It was then stored in a -80℃ freezer (NF-400SF, HFC, Japan) until further analysis.
Analysis items and Methods
Body weight measurement
During the experiment, body weight was measured under the same environment. It was measured every Monday and Thursday at 9:00 AM (twice a week).
Western blot analysis
To determine protein levels, liver tissue was homogenized in 0.5 M EDTA (Duksan, Korea), lysis buffer (Gendepot, R4200-100, USA), and phosphatase inhibitor 100 x (Gendepot, P3200-001, USA). After homogenization, each sample was centrifuged at 1,200 x g for 10 minutes. Supernatants were collected and subjected to protein concentration measurement using Bradford protein assay method. Briefly, standard BSA or supernatant (1 ㎕) was mixed with 1 ml of Bradford reagent. A 200 ㎕ of the mix was then placed in an ELISA plate to measure the absorbance value at wavelength of 595 nm. After protein concentration measurement, proteins were mixed with Laemmli sample buffer and boiled for 5 min. Protein samples (20μl each) were then loaded into 10% SDS-PAGE gel and run at 100 V to separate the proteins. After electrophoresis, proteins were transferred onto polyvinylidene difluoride (PVDF) membranes by electro-blotting at 100 V at room temperature for 1 h (± 20~30 min). The membranes were incubated with 5% skim milk blocking buffer (Skim milk block buffer; TBST+5% skim milk) for 1 h followed by incubation with primary antibodies at 4℃ overnight. Primary antibodies were prepared by diluting in 5% skim milk. The following primary antibodies were used: HSP70 1:2,000 (Abcam, UK), Bcl-2 1:200 (Santa cruz, USA), p53 1: 1,000 (Abcam, UK), and caspase-3 1:1,000 (Cell signaling, USA). After washing 5 times (10 min each) with TBST buffer (50 mM Tris-Hcl, 150 mM NaCl and 0.05%, Tween 20), membranes were incubated with respective secondary antibodies at room temperature for 1 h. For HSP70 1:5,000, goat-anti rabbit secondary antibody (Santa Cruz, USA) was used. For Bcl-2 1:2,000, goat-anti rabbit secondary antibody (Santa Cruz, USA) was used. For p53 1:5,000, goat-anti mouse secondary antibody (Santa Cruz, USA) was used. For caspase-3 1:3,000, goat-anti rabbit secondary antibody (Santa Cruz, USA). After rinsing with TBST (5% tween-20) 5 times (10 min each), the membranes were incubated with ECL reagent and developed onto X-ray film. Band intensities were measured with Image J (NIH, Ver. 1.47t, USA). Their intensities were normalized to the level of β-actin as loading control.
Cortisol analysis
Blood cortisol concentrations were measured using an ELISA kit (MyBioSource, CANADA). Briefly, blood samples were centrifuged at 1,000 rpm for 10 minutes and left at room temperature for 20 minutes after diluting the sample with reagent provided by the kit in accordance with the manufacturer’s instructions. Both standard and sample dilutions were performed in 96-well plates. They were incubated at 37℃ after mixing 100 ㎕ for 90 minutes in a shaker. Washing was then performed with buffer three times, followed by the addition of 400 ㎕ to each well after mixing. The plate was turned upside down to remove the wash buffer. Then 100 ㎕ of prepared antibody was added to each well and incubated at 37℃ with shaking for 60 minutes. The plate was then washed with washing buffer three times. Cortisol enzyme-conjugate mixed with buffer (100 ㎕) was then added to each well followed by incubation at 37℃ for 30 minutes with shaking. Finally, washing was performed using 400 ㎕ of washing buffer five times. The plate was turned upside down to remove the wash buffer. After adding 100 ㎕ of a dark color reagent, the 96-well plate was placed on a shaker for mixing, followed by addition of 100 ㎕ of color reagent C. After 30 minutes of mixing, the absorbance value was measured at wavelength of 450 nm on an ELISA reader (Tecan Infinite, F50, Austria).
Statistics
All data analysis in this study were performed with SPSS/PC+20.0, a statistical program designed for Window-based PCs. All test data were presented in averages with standard errors. One-way analysis of variance (ANOVA) was conducted to verify significant differences among groups. In case of an item with significant difference, post verification was carried out using the Least Significant Different method. All significance levels were set at p < 0.05.
RESULTS
Body weight gain
Results of body weight gain are shown in Figure 2. As shown in Figure 2, there was no significant difference in body weight gain among the three groups. However, the body weight gains in CON and RS tended to be smaller compared to that of RSVE group.
Protein expression level
Apoptosis-related protein expression levels of HSP70 (Figure 3), p53 (Figure 4), and Bcl-2 (Figure 5) were not statistically different among the three groups. However, caspase-3 protein expression (Figure 6) was significantly (p < 0.05) higher in RS group compared to that in the other two groups.

Difference in heat shock protein 70 (HSP70) protein expression. Bars are mean ± standard error. CON and white bar: control group; RS and striped pattern bar: restraint stress group; RSVE and black bar: restraint stress and voluntary exercise group.

Difference in p53 protein expression. Bars are mean ± standard error. CON and white bar: control group; RS and striped pattern bar: restraint stress group; RSVE and black bar: restraint stress and voluntary exercise group.
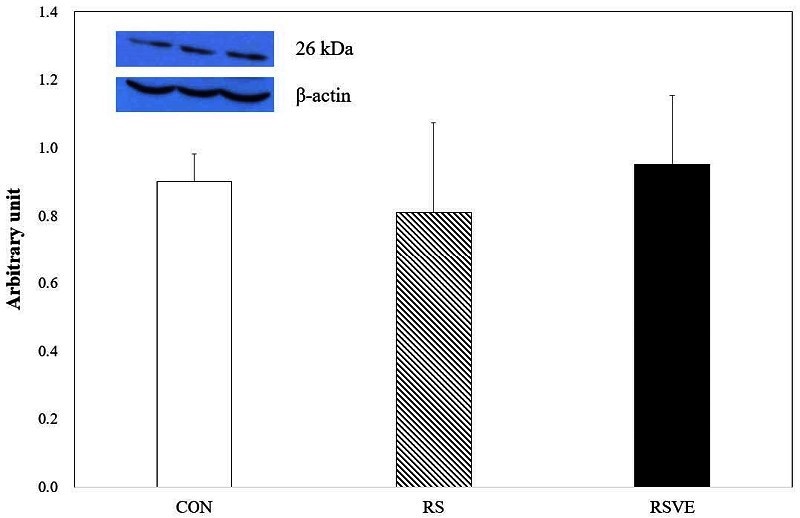
Difference in Bcl-2 protein expression. Bars are mean ± standard error. CON and white bar: control group; RS and striped pattern bar: restraint stress group; RSVE and black bar: restraint stress and voluntary exercise group.
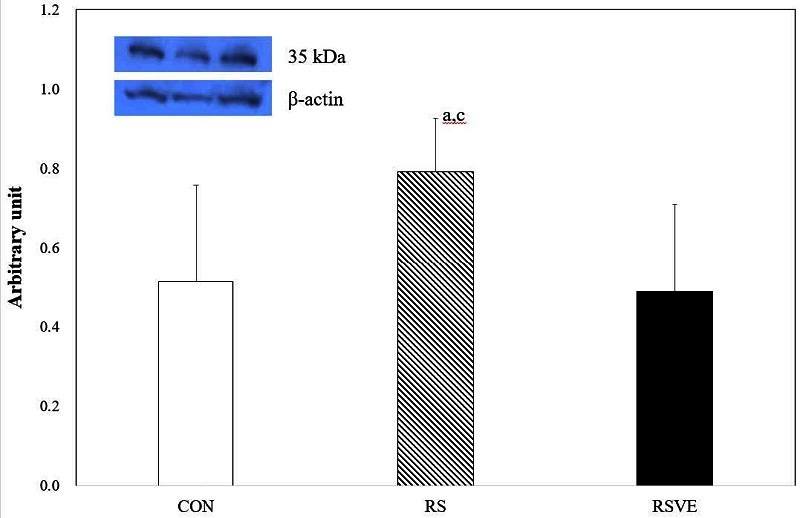
Difference in caspase-3 protein expression. Bars are mean ± standard error. CON and white bar: control group; RS and striped pattern bar: restraint stress group; RSVE and black bar: restraint stress and voluntary exercise group. a: significantly different from CON, p < 0.05; c: significantly different from RSVE, p < 0.05.
Blood cortisol level
Results of blood cortisol concentrations are shown in Figure 7. The highest concentration of blood cortisol was found in the RS group. The lowest blood cortisol level was found in the RSVE group with statistical significance (p < 0.05). Blood cortisol level in the RSVE was also significantly (p < 0.05) lower than that in CON.

Difference in blood cortisol concentration. Bars are mean ± standard error. CON and white bar: control group; RS and striped pattern bar: restraint stress group; RSVE and black bar: restraint stress and voluntary exercise group. a: significantly different from CON, p < 0.05; b: significantly different form RS, p < 0.05; c: significantly different from RSVE, p < 0.05.
DISCUSSION
In this study, we examined the effect of voluntary exercise on apoptosis and blood cortisol level using chronic restraint stress mice model. It has been reported that relatively gentle exercise for C57BL/6 mice without electrical stimulation during treadmill running with voluntary walking speed may prevent contamination of experimental data23. In addition, rats with forced-treadmill exercise have shown more complicated theta wave in the brain and heart rate than rats with voluntary exercise rats25. Therefore, we used a voluntary exercise device in this study.
In general, chronic stress can cause weight loss, especially when restraint stress is loaded. Chronic stress also inhibits weight gain during mice breeding26. After restraint stress for 14 days, body weight gain is suppressed from the third day of the experiment. Then a few amount of body weight is recovered after the recovery periods27. However, in the present study, there was no difference in weight gain in RS. However, RSVE showed approximately 5% of weight gain with increased amount of skeletal muscles with exercise training. It has been reported that voluntary running exercise can increase the amount of lower limbs’ skeletal muscle28, 29. These results implies that participation of voluntary exercise in a stressful situation that may prevent weight loss. In addition, voluntary exercise can serve as a controller for someone who suffers from many kinds of stress that can cause weight loss despite of the same amount of meal intake30.
Restraint stress can inhibit the biosynthesis of melanin in the skin13, weaken the hippocampus and cortical GABAergic neurotransmitters31, and induce depression in pregnant rats32. It has been reported that heat shock protein 70 (HSP70) is increased under stress situations33. Under lipid peroxidation condition, HSP70 level is increased dramatically in a short period of time34. In this study, there was no difference in the expression levels of HSP70 among the three groups, although its level was increased by RS. HSP70 can suppress stress-induced myocardial apoptosis through interaction with FAF-135 and stress protein marker HSP 72, resulted in apoptosis suppression in exercised rats9. Our results suggest that chronic restraint stress can trigger stress, the lead cause of apoptosis. However, there was no difference among the three groups. Therefore, we carefully analyzed the expression levels of apoptosis-related proteins.
Meanwhile, p53 protein is a well-known tumor suppressor. Bcl-2 protein expression is increased when the expression of p53 protein is suppressed36. Higgins et al.37 have reported that exercise can increase p53 protein expression in lung cancer tumor tissue and induce apoptosis in lung cancer. However, p53 protein expression is suppressed even with exercise training38. In addition, in stress-induced mice, despite receiving various kinds of vitamins without considering exercise training, only a tendency of p53 reduction is observed whereas the phosphorylation of p53 is increased by exercise39. However, there was no significant difference in the levels of p53 among the three groups in the present study, although there was a tendency of a higher expression of p53 in RS and lower expression in RSVE.
Anti-apoptosis bcl-2 protein can refrain from apoptosis. When morphine-dependent apoptosis in induced, voluntary exercise or exercise participation can increase the level of bcl-2 protein expression, thus suppresses apoptosis20. However, its expression is not increased by acute high-intensity swimming exercise12. Various kinds of stress can also suppress hippocampal bcl-2 level. Therefore, we hypothesized that bcl-2 level would be reduced under stressful situations but increased by voluntary exercise. However, in this study, bcl-2 showed only a tendency of increase without statistical significance. A significant decrease in bcl-2 level has been observed under restraint stress in ovarian cells3. However, long-term swimming exercise does not change protein expression level of bcl-24. In addition, bcl-2 level is increased by treadmill exercise41. Considering those reports, blc-2 expression levels do not always show the same results. Therefore, future research studies with various methodological approaches are needed to determine the role of blc-2 in stress.
Caspase-3 is a protein that directly induces apoptosis36. An eight-week swimming exercise training has resulted in significantly reduced apoptosis and apoptosis marker expression in the hippocampus. In addition, swimming training has reduced casepase-3 protein expression42. As previously mentioned, exercise can result in effective suppression of apoptosis43. Kim et al.21 have reported that rat pups born from diabetic rats who performed treadmill exercise have shown suppression of apoptosis. This study limitation in studying the expression level of caspase-3 is that cleaved caspase-3 expression levels are usually presented in the results as its protein expression level instead of the full-length caspase-3. This study also showed that caspase-3 protein expression was suppressed by voluntary exercise in RSVE. On the contrary, it showed higher expression by restraint stress in RS. These results might be due to the fact that, after 1 h of restraint stress, rats freely participated the voluntary exercise. This might have affected caspase-3 protein expression. It has been reported that prolonged restraint stress load can result in the increase of caspase-3 and apoptosis in rats44.
Cortisol, a typical stress hormone, is secreted from the adrenal cortex of the kidney. Cortisol can respond to stimuli such as stress. In this study, cortisol was increased significantly by restraint stress. However, it showed significantly lower level during voluntary exercise after restraint stress. Interestingly, RSVE had significantly lower cortisol levels than CON. It has been reported that cortisol level is significantly increased by restraint stress and the trend has started from the first day of restraint5. When Cynomolgus monkey is tied in a chair to limit its mobility, its cortisol concentration is increased45. Similarly, as a stress response of bottlenose dolphin, its cortisol level is continuously increased until the time it is discharged from the initial capture46. Serum cortisol concentration is also increased 5-fold compared to that in the control group after one hour of restraint47.
The changes of serum cortisol concentration in Wistar rats under forced swim and exercise restraint situations have been studied previously2. It was found that serum cortisol concentrations were significantly increased under the restraint condition and the forced swimming exercise situation. Additional forced swimming exercise after restraint stress additionally increased the cortisol levels, although not statistically significant. These results suggested that restraint brings more stress than forced swimming exercise. In addition, it has been reported that cortisol level is significantly increased by resistance training48 or intermittent exercise training49. However, these researches were focused on relatively high intensity exercise that could induce exhaustion. Under long term stressful situations, a voluntary exercise does not have significant effect on cortisol level50. Therefore, under specific stress situations, participation of voluntary exercise may be one of the way to reduce stress.
In summary, the results of the present study suggest that restraint stress for a long period of time is likely to accelerate aging and induce apoptosis. In addition, considering the changes of cortisol concentrations, long-term stress exposure can increase the prevalence of various metabolic disorders. An alternative way to reduce stress, voluntary exercise can bring positive effect on health because it inhibits apoptosis and suppress cortisol level. Further research studies are needed to determine the effect of both nutritional intake and exercises on health under a variety of stressful situations.
References
Laurence A, Houdelier C, Calandreau L, Arnould C, Favreau-Peigné A, Leterrier C, Boissy A, Lumineau S. Environmental enrichment reduces behavioural alterations induced by chronicstress in Japanese quail. J Animal. 2015; 9: 331-8.
. Laurence A., Houdelier C., Calandreau L., Arnould C., Favreau-Peigné A., Leterrier C., Boissy A., Lumineau S.. Environmental enrichment reduces behavioural alterations induced by chronicstress in Japanese quail. J Animal 2015;9:331–8. 10.1017/S1751731114002523.Jameel MK, Joshi AR, Dawane J, Padwal M, Joshi A, Pandit VA, Melinkeri R. Effect of various physical stress models on serum cortisol level in wistar rats. J Clin Diagn Res. 2014; 8: 181-3.
. Jameel MK., Joshi AR., Dawane J., Padwal M., Joshi A., Pandit VA., Melinkeri R.. Effect of various physical stress models on serum cortisol level in wistar rats. J Clin Diagn Res 2014;8:181–3. 10.7860/jcdr/2014/7210.4116.Liang B, Wei DL, Cheng YN, Yuan HJ, Lin J, Cui XZ, Luo MJ, Tan JH. Restraint stress impairs oocyte developmental potential in mice: role of CRH-induced apoptosis of ovarian cells. Biol Reprod. 2013; 89: 64.
. Liang B., Wei DL., Cheng YN., Yuan HJ., Lin J., Cui XZ., Luo MJ., Tan JH.. Restraint stress impairs oocyte developmental potential in mice: role of CRH-induced apoptosis of ovarian cells. Biol Reprod 2013;89:64. 10.1095/biolreprod.113.110619. 23884643.Roberts CJ, Campbell IC, Troop N. Increases in weight during chronic stress are partially associated with a switch in food choice towards increased carbohydrate and saturated fat intake. Eur Eat Disord Rev. 2014; 22: 77-82.
. Roberts CJ., Campbell IC., Troop N.. Increases in weight during chronic stress are partially associated with a switch in food choice towards increased carbohydrate and saturated fat intake. Eur Eat Disord Rev 2014;22:77–82. 10.1002/erv.2264. 24123563.Shirasaki Y, Yoshioka N, Kanazawa K, Maekawa T, Horikawa T, Hayashi T. Effect of physical restraint on glucose tolerance in cynomolgus monkeys. J Med Primatol. 2013; 42: 165-8.
. Shirasaki Y., Yoshioka N., Kanazawa K., Maekawa T., Horikawa T., Hayashi T.. Effect of physical restraint on glucose tolerance in cynomolgus monkeys. J Med Primatol 2013;42:165–8. 10.1111/jmp.12039. 23802316.Hare BD, Beierle JA, Toufexis DJ, Hammack SE, Falls WA. Exercise-associated changes in the corticosterone response to acute restraint stress: evidence for increased adrenal sensitivity and reduced corticosterone response duration. Neuropsychopharmacology. 2013; 3: 1262-9.
. Hare BD., Beierle JA., Toufexis DJ., Hammack SE., Falls WA.. Exercise-associated changes in the corticosterone response to acute restraint stress: evidence for increased adrenal sensitivity and reduced corticosterone response duration. Neuropsychopharmacology 2013;3:1262–9. 10.1038/npp.2013.329.Han TK, Lee JK, Leem YH. Chronic exercise prevents repeated restraint stress-provoked enhancement of immobility in forced swimming test in ovariectomized mice. Metab Brain Dis. 2015; 30: 711-8.
. Han TK., Lee JK., Leem YH.. Chronic exercise prevents repeated restraint stress-provoked enhancement of immobility in forced swimming test in ovariectomized mice. Metab Brain Dis 2015;30:711–8. 10.1007/s11011-014-9624-2. 25344674.Livingston-Thomas JM, McGuire EP, Doucette TA, Tasker RA. Voluntary forced use of the impaired limb following stroke facilitates functional recovery in the rat. Behav Brain Res. 2014; 261: 210-9.
. Livingston-Thomas JM., McGuire EP., Doucette TA., Tasker RA.. Voluntary forced use of the impaired limb following stroke facilitates functional recovery in the rat. Behav Brain Res 2014;261:210–9. 10.1016/j.bbr.2013.12.032. 24388978.Chang CK, Chou W, Lin HJ, Huang YC, Tang LY, Lin MT, Chang CP. Exercise preconditioning protects against spinal cord injury in rats by upregulating neuronal and astroglial heat shock protein 72. Int J Mol Sci. 2014; 15: 19018-36.
. Chang CK., Chou W., Lin HJ., Huang YC., Tang LY., Lin MT., Chang CP.. Exercise preconditioning protects against spinal cord injury in rats by upregulating neuronal and astroglial heat shock protein 72. Int J Mol Sci 2014;15:19018–36. 10.3390/ijms151019018. 25334068.Kwak HB, Lee Y, Kim JH, Van Remmen H, Richardson AG, Lawler JM. MnSOD Overexpression Reduces Fibrosis and Pro-Apoptotic Signaling in the Aging Mouse Heart. J Gerontol A Biol Sci Med Sci. 2015; 70: 533-44.
. Kwak HB., Lee Y., Kim JH., Van Remmen H., Richardson AG., Lawler JM.. MnSOD Overexpression Reduces Fibrosis and Pro-Apoptotic Signaling in the Aging Mouse Heart. J Gerontol A Biol Sci Med Sci 2015;70:533–44. 10.1093/gerona/glu090. 25016531.Chen KC, Peng CC, Hsieh CL, Peng RY. Exercise ameliorates renal cell apoptosis in chronic kidney disease by intervening in the intrinsic and the extrinsic apoptotic pathways in a rat model. Evid Based Complement Alternat Med. 2013; 2013: 368450.
. Chen KC., Peng CC., Hsieh CL., Peng RY.. Exercise ameliorates renal cell apoptosis in chronic kidney disease by intervening in the intrinsic and the extrinsic apoptotic pathways in a rat model. Evid Based Complement Alternat Med 2013;2013:368450. 10.1155/2013/368450. 24106522.Ding Y, Chang C, Xie L, Chen Z, Ai H. Intense exercise can cause excessive apoptosis and synapse plasticity damage in rat hippocampus through Ca(2+) overload and endoplasmic reticulum stress-induced apoptosis pathway. J Chin Med. 2014; 127: 3265-71.
. Ding Y., Chang C., Xie L., Chen Z., Ai H.. Intense exercise can cause excessive apoptosis and synapse plasticity damage in rat hippocampus through Ca(2+) overload and endoplasmic reticulum stress-induced apoptosis pathway. J Chin Med 2014;127:3265–71.Krüger K, Mooren FC. Exercise-induced leukocyte apoptosis. Exerc Immunol Rev. 2014; 20: 117-34.
. Krüger K., Mooren FC.. Exercise-induced leukocyte apoptosis. Exerc Immunol Rev 2014;20:117–34. 24974724.Pang S, Wu H, Wang Q, Cai M, Shi W, Shang J. Chronic stress suppresses the expression of cutaneous hypothalamic-pituitary-adrenocortical axis elements and melanogenesis. PLoS One. 2014; 9: e98283.
. Pang S., Wu H., Wang Q., Cai M., Shi W., Shang J.. Chronic stress suppresses the expression of cutaneous hypothalamic-pituitary-adrenocortical axis elements and melanogenesis. PLoS One 2014;9e98283. 10.1371/journal.pone.0098283.Uribe RM, Jaimes-Hoy L, Ramírez-Martínez C, García- Vázquez A, Romero F, Cisneros M, Cote-Vélez A, Charli JL, Joseph-Bravo P. Voluntary exercise adapts the hypothalamus-pituitary-thyroid axis in male rats. Endocrinology. 2014; 155: 2020-30.
. Uribe RM., Jaimes-Hoy L., Ramírez-Martínez C., García-Vázquez A., Romero F., Cisneros M., Cote-Vélez A., Charli JL., Joseph-Bravo P.. Voluntary exercise adapts the hypothalamus-pituitary-thyroid axis in male rats. Endocrinology 2014;155:2020–30. 10.1210/en.2013-1724. 24605825.Castilla-Ortega E, Rosell-Valle C, Pedraza C, Rodríguez de Fonseca F, Estivill-Torrús G, Santín LJ. Voluntary exercise followed by chronic stress strikingly increases mature adult-born hippocampal neurons and prevents stress-induced deficits in ‘what-when-where’ memory. Neurobiol Learn Mem. 2014; 109: 62-73.
. Castilla-Ortega E., Rosell-Valle C., Pedraza C., Rodríguez de Fonseca F., Estivill-Torrús G., Santín LJ.. Voluntary exercise followed by chronic stress strikingly increases mature adult-born hippocampal neurons and prevents stress-induced deficits in ‘what-when-where’ memory. Neurobiol Learn Mem 2014;109:62–73. 10.1016/j.nlm.2013.12.001. 24333647.Luo B, Xiang D, Nieman DC, Chen P. The effects of moderate exercise on chronic stress-induced intestinal barrier dysfunction and antimicrobial defense. Brain Behav Immun. 2014; 39: 99-106.
. Luo B., Xiang D., Nieman DC., Chen P.. The effects of moderate exercise on chronic stress-induced intestinal barrier dysfunction and antimicrobial defense. Brain Behav Immun 2014;39:99–106. 10.1016/j.bbi.2013.11.013. 24291325.Radahmadi M, Alaei H, Sharifi MR, Hosseini N. The effect of synchronized forced running with chronic stress on short, mid and long-term memory in rats. Asian J Sports Med. 2013; 4: 54-62.
. Radahmadi M., Alaei H., Sharifi MR., Hosseini N.. The effect of synchronized forced running with chronic stress on short, mid and long-term memory in rats. Asian J Sports Med 2013;4:54–62. 10.5812/asjsm.34532. 23785577.Uysal N, Kiray M, Sisman A, Camsari U, Gencoglu C, Baykara B, Cetinkaya C, Aksu I. Effects of voluntary and involuntary exercise on cognitive functions, and VEGF and BDNF levels in adolescent rats. Biotech Histochem. 2014; 1-14.
. Uysal N., Kiray M., Sisman A., Camsari U., Gencoglu C., Baykara B., Cetinkaya C., Aksu I.. Effects of voluntary and involuntary exercise on cognitive functions, and VEGF and BDNF levels in adolescent rats. Biotech Histochem 2014;:1–14. 10.3109/10520295.2014.946968.Mokhtari-Zaer A, Ghodrati-Jaldbakhan S, Vafaei AA, Miladi-Gorji H, Akhavan MM, Bandegi AR, Rashidy-Pour A. Effects of voluntary and treadmill exercise on spontaneous withdrawal signs, cognitive deficits and alterations in apoptosis-associated proteins in morphine-dependent rats. Behav Brain Res. 2014; 271: 160-70.
. Mokhtari-Zaer A., Ghodrati-Jaldbakhan S., Vafaei AA., Miladi-Gorji H., Akhavan MM., Bandegi AR., Rashidy-Pour A.. Effects of voluntary and treadmill exercise on spontaneous withdrawal signs, cognitive deficits and alterations in apoptosis-associated proteins in morphine-dependent rats. Behav Brain Res 2014;271:160–70. 10.1016/j.bbr.2014.05.061. 24906198.Kim YH, Sung YH, Lee HH, Ko IG, Kim SE, Shin MS, Kim BK. Postnatal treadmill exercise alleviates short-term memory impairment by enhancing cell proliferation and suppressing apoptosis in the hippocampus of ratpups born to diabetic rats. J Exerc Rehabil. 2014; 10: 209-17.
. Kim YH., Sung YH., Lee HH., Ko IG., Kim SE., Shin MS., Kim BK.. Postnatal treadmill exercise alleviates short-term memory impairment by enhancing cell proliferation and suppressing apoptosis in the hippocampus of ratpups born to diabetic rats. J Exerc Rehabil 2014;10:209–17. 10.12965/jer.140145. 25210695.Ogbonmwan YE, Schroeder JP, Holmes PV, Weinshenker D. The effects of post-extinction exercise on cocaine-primed and stress-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl). 2015; 232: 1395-403.
. Ogbonmwan YE., Schroeder JP., Holmes PV., Weinshenker D.. The effects of post-extinction exercise on cocaine-primed and stress-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2015;232:1395–403. 10.1007/s00213-014-3778-9. 25358851.Conner JD, Wolden-Hanson T, Quinn LS. Assessment of murine exercise endurance without the use of a shock grid: an alternative to forced exercise. J Vis Exp. 2014; 90: e51846.
. Conner JD., Wolden-Hanson T., Quinn LS.. Assessment of murine exercise endurance without the use of a shock grid: an alternative to forced exercise. J Vis Exp 2014;90e51846. 10.3791/51846.Fan X, Li D, Lichti CF, Green TA. Dynamic proteomics of nucleus accumbens in response to acute psychologicalstress in environmentally enriched and isolated rats. PLoS One. 2013; 8: e73689.
. Fan X., Li D., Lichti CF., Green TA.. Dynamic proteomics of nucleus accumbens in response to acute psychologicalstress in environmentally enriched and isolated rats. PLoS One 2013;8e73689. 10.1371/journal.pone.0073689.Li JY, Kuo TB, Yen JC, Tsai SC, Yang CC. Voluntary and involuntary running in the rat show different patterns of theta rhythm, physical activity, and heart rate. J Neurophysiol. 2014; 111: 2061-70.
. Li JY., Kuo TB., Yen JC., Tsai SC., Yang CC.. Voluntary and involuntary running in the rat show different patterns of theta rhythm, physical activity, and heart rate. J Neurophysiol 2014;111:2061–70. 10.1152/jn.00475.2013. 24623507.Zhang W, Hetzel A, Shah B, Atchley D, Blume SR, Padival MA, Rosenkranz JA. Greater physiological and behavioral effects of interrupted stress pattern compared to daily restraint stress in rats. PLoS One. 2014; 9: e102247.
. Zhang W., Hetzel A., Shah B., Atchley D., Blume SR., Padival MA., Rosenkranz JA.. Greater physiological and behavioral effects of interrupted stress pattern compared to daily restraint stress in rats. PLoS One 2014;9e102247. 10.1371/journal.pone.0102247.Babenko O, Golubov A, Ilnytskyy Y, Kovalchuk I, Metz GA. Genomic and epigenomic responses to chronic stress involve miRNA-mediated programming. PLoS One. 2012; 7: e29441.
. Babenko O., Golubov A., Ilnytskyy Y., Kovalchuk I., Metz GA.. Genomic and epigenomic responses to chronic stress involve miRNA-mediated programming. PLoS One 2012;7e29441. 10.1371/journal.pone.0029441.Lockwood CM, Moon JR, Tobkin SE, Walter AA, Smith AE, Dalbo VJ, Cramer JT, Stout JR. Minimal nutrition intervention with high-protein/low-carbohydrate and low-fat, nutrient-dense food supplement improves body composition and exercise benefits in overweight adults: A randomized controlled trial. Nutr Metab (Lond). 2008; 21: 11.
. Lockwood CM., Moon JR., Tobkin SE., Walter AA., Smith AE., Dalbo VJ., Cramer JT., Stout JR.. Minimal nutrition intervention with high-protein/low-carbohydrate and low-fat, nutrient-dense food supplement improves body composition and exercise benefits in overweight adults: A randomized controlled trial. Nutr Metab (Lond) 2008;21:11. 10.1186/1743-7075-5-11.Momken I, Lechêne P, Koulmann N, Fortin D, Mateo P, Doan BT, Hoerter J, Bigard X, Veksler V, Ventura-Clapier R. Impaired voluntary running capacity of creatine kinase-deficient mice. J Physiol. 2005; 565: 951-64.
. Momken I., Lechêne P., Koulmann N., Fortin D., Mateo P., Doan BT., Hoerter J., Bigard X., Veksler V., Ventura-Clapier R.. Impaired voluntary running capacity of creatine kinase-deficient mice. J Physiol 2005;565:951–64. 10.1113/jphysiol.2005.086397. 15831533.Patki G, Solanki N, Atrooz F, Allam F, Salim S. Depression, anxiety-like behavior and memory impairment are associated with increased oxidative stress and inflammation in a rat model of social stress. Brain Res. 2013; 1539: 73-86.
. Patki G., Solanki N., Atrooz F., Allam F., Salim S.. Depression, anxiety-like behavior and memory impairment are associated with increased oxidative stress and inflammation in a rat model of social stress. Brain Res 2013;1539:73–86. 10.1016/j.brainres.2013.09.033. 24096214.Wisłowska-Stanek A, Lehner M, Skórzewska A, Krząścik P, Maciejak P, Szyndler J, Ziemba A, Płaźnik A. Changes in the brain expression of alpha-2 subunits of the GABA-A receptor after chronic restraint stress in low- and high-anxiety rats. Behav Brain Res. 2013; 253: 337-45.
. Wisłowska-Stanek A., Lehner M., Skórzewska A., Krząścik P., Maciejak P., Szyndler J., Ziemba A., Płaźnik A.. Changes in the brain expression of alpha-2 subunits of the GABA-A receptor after chronic restraint stress in low- and high-anxiety rats. Behav Brain Res 2013;253:337–45. 10.1016/j.bbr.2013.07.042. 23916758.Maghsoudi N, Ghasemi R, Ghaempanah Z, Ardekani AM, Nooshinfar E, Tahzibi A. Effect of chronic restraint stress on HPA axis activity and expression of BDNF and Trkb in the hippocampus of pregnant rats: possible contribution in depression during pregnancy and postpartum period. Basic Clin Neurosci. 2014; 5: 131-7.
. Maghsoudi N., Ghasemi R., Ghaempanah Z., Ardekani AM., Nooshinfar E., Tahzibi A.. Effect of chronic restraint stress on HPA axis activity and expression of BDNF and Trkb in the hippocampus of pregnant rats: possible contribution in depression during pregnancy and postpartum period. Basic Clin Neurosci 2014;5:131–7. 25337371.Juhász K, Thuenauer R, Spachinger A, Duda E, Horváth I, Vígh L, Sonnleitner A, Balogi Z. Lysosomal rerouting of Hsp70 trafficking as a potential immune activating tool for targeting melanoma. Curr Pharm Des. 2013; 19: 430-40.
. Juhász K., Thuenauer R., Spachinger A., Duda E., Horváth I., Vígh L., Sonnleitner A., Balogi Z.. Lysosomal rerouting of Hsp70 trafficking as a potential immune activating tool for targeting melanoma. Curr Pharm Des 2013;19:430–40. 10.2174/138161213804143644. 22920897.Grune T, Catalgol B, Licht A, Ermak G, Pickering AM, Ngo JK, Davies KJ. HSP70 mediates dissociation and reassociation of the 26S proteasome during adaptation to oxidative stress. Free Radic Biol Med. 2011; 51: 1355-64.
. Grune T., Catalgol B., Licht A., Ermak G., Pickering AM., Ngo JK., Davies KJ.. HSP70 mediates dissociation and reassociation of the 26S proteasome during adaptation to oxidative stress. Free Radic Biol Med 2011;51:1355–64. 10.1016/j.freeradbiomed.2011.06.015. 21767633.Gao X, Liu W, Huang L, Zhang T, Mei Z, Wang X, Gong J, Zhao Y, Xie F, Ma J, Qian L. HSP70 inhibits stress-induced cardiomyocyte apoptosis by competitively binding to FAF1. Cell Stress Chaperones. 2015; 20: 653-61.
. Gao X., Liu W., Huang L., Zhang T., Mei Z., Wang X., Gong J., Zhao Y., Xie F., Ma J., Qian L.. HSP70 inhibits stress-induced cardiomyocyte apoptosis by competitively binding to FAF1. Cell Stress Chaperones 2015;20:653–61. 10.1007/s12192-015-0589-9. 25935138.Seo H, Lee NH, Ryu S. Antioxidant and antiapoptotic effects of pine needle powder ingestion and endurance training in high cholesterol-fed rats. J Exerc Nutr Biochem. 2014; 18: 301-9.
. Seo H., Lee NH., Ryu S.. Antioxidant and antiapoptotic effects of pine needle powder ingestion and endurance training in high cholesterol-fed rats. J Exerc Nutr Biochem 2014;18:301–9.Higgins KA, Park D, Lee GY, Curran WJ, Deng X. Exercise-induced lung cancer regression: mechanistic findings from a mouse model. Cancer. 2014; 120: 3302-10.
. Higgins KA., Park D., Lee GY., Curran WJ., Deng X.. Exercise-induced lung cancer regression: mechanistic findings from a mouse model. Cancer 2014;120:3302–10. 10.1002/cncr.28878. 24989479.Kao CL, Tsai KL, Cheng YY, Kuo CH, Lee SD, Chan RC. Vestibular rehabilitation ameliorates chronic dizziness through the SIRT1 axis. Front Aging Neurosci. 2014; 6: 27.
. Kao CL., Tsai KL., Cheng YY., Kuo CH., Lee SD., Chan RC.. Vestibular rehabilitation ameliorates chronic dizziness through the SIRT1 axis. Front Aging Neurosci 2014;6:27. 10.3389/fnagi.2014.00027. 24624081.Wawrzyniak A, Górnicka M, Hamułka J, Gajewska M, Drywień M, Pierzynowska J, Gronowska-Senger A. α-Tocopherol, ascorbic acid, and β-carotene protect against oxidative stress but reveal no direct influence on p53 expression in rats subjected to stress. Nutr Res. 2013; 33: 868-75.
. Wawrzyniak A., Górnicka M., Hamułka J., Gajewska M., Drywień M., Pierzynowska J., Gronowska-Senger A.. α-Tocopherol, ascorbic acid, and β-carotene protect against oxidative stress but reveal no direct influence on p53 expression in rats subjected to stress. Nutr Res 2013;33:868–75. 10.1016/j.nutres.2013.07.001. 24074745.Ziolkowski W, Flis DJ, Halon M, Vadhana DM, Olek RA, Carloni M, Antosiewicz J, Kaczor JJ, Gabbianelli R. Prolonged swimming promotes cellular oxidative stress and p66Shc phosphorylation, but does not induce oxidative stress in mitochondria in the rat heart. Free Rad Res. 2015; 49: 7-16.
. Ziolkowski W., Flis DJ., Halon M., Vadhana DM., Olek RA., Carloni M., Antosiewicz J., Kaczor JJ., Gabbianelli R.. Prolonged swimming promotes cellular oxidative stress and p66Shc phosphorylation, but does not induce oxidative stress in mitochondria in the rat heart. Free Rad Res 2015;49:7–16. 10.3109/10715762.2014.968147.Kim DY, Jung SY, Kim CJ, Sung YH, Kim JD. Treadmill exercise ameliorates apoptotic cell death in the retinas of diabetic rats. Mol Med Rep. 2013; 7: 1745-50.
. Kim DY., Jung SY., Kim CJ., Sung YH., Kim JD.. Treadmill exercise ameliorates apoptotic cell death in the retinas of diabetic rats. Mol Med Rep 2013;7:1745–50. 10.3892/mmr.2013.1439. 23620139.Mourão FA, Leite HR, de Carvalho LE, Ferreira E Vieira TH, Pinto MC, de Castro Medeiros D, Andrade IL, Gonçalves DF, Pereira GS, Dutra Moraes MF, Massensini AR. Neuroprotective effect of exercise in rat hippocampal slices submitted to in vitro ischemia is promoted by decrease of glutamate release and pro-apoptotic markers. J Neurochem. 2014; 131: 65-73.
. Mourão FA., Leite HR., de Carvalho LE., Ferreira E., Vieira TH., Pinto MC., de Castro Medeiros D., Andrade IL., Gonçalves DF., Pereira GS., Dutra Moraes MF., Massensini AR.. Neuroprotective effect of exercise in rat hippocampal slices submitted to in vitro ischemia is promoted by decrease of glutamate release and pro-apoptotic markers. J Neurochem 2014;131:65–73. 10.1111/jnc.12786. 24903976.Kwak HB. Effects of aging and exercise training on apoptosis in the heart. J Exerc Rehabil. 2013; 9: 212-9.
. Kwak HB.. Effects of aging and exercise training on apoptosis in the heart. J Exerc Rehabil 2013;9:212–9. 10.12965/jer.130002. 24278863.Lehner M, Wisłowska-Stanek A, Skórzewska A, Płaźnik A. Chronic restraint increases apoptosis in the hippocampus of rats with high responsiveness to fear stimuli. Neurosci Lett. 2015; 586: 55-9.
. Lehner M., Wisłowska-Stanek A., Skórzewska A., Płaźnik A.. Chronic restraint increases apoptosis in the hippocampus of rats with high responsiveness to fear stimuli. Neurosci Lett 2015;586:55–9. 10.1016/j.neulet.2014.12.011. 25486591.Gong S, Miao YL, Jiao GZ, Sun MJ, Li H, Lin J, Luo MJ, Tan JH. Dynamics and correlation of serum cortisol and corticosterone under different physiological or stressful conditions in mice. PLoS One. 2015; 10: e0117503.
. Gong S., Miao YL., Jiao GZ., Sun MJ., Li H., Lin J., Luo MJ., Tan JH.. Dynamics and correlation of serum cortisol and corticosterone under different physiological or stressful conditions in mice. PLoS One 2015;10e0117503. 10.1371/journal.pone.0117503.Fair PA, Schaefer AM, Romano TA, Bossart GD, Lamb SV, Rief JS. Stress response of wild bottlenose dolphins (Tursiops truncatus) during capture-release health assessment studies. Gen Comp Endocrinol. 2014; 206: 203-12.
. Fair PA., Schaefer AM., Romano TA., Bossart GD., Lamb SV., Rief JS.. Stress response of wild bottlenose dolphins (Tursiops truncatus) during capture-release health assessment studies. Gen Comp Endocrinol 2014;206:203–12. 10.1016/j.ygcen.2014.07.002. 25019655.Zhao LH, Cui XZ, Yuan HJ, Liang B, Zheng LL, Liu YX, Luo MJ, Tan JH. Restraint stress inhibits mouse implantation: temporal window and the involvement of HB-EGF, estrogen and progesterone. PLoS One. 2013; 8: e80472.
. Zhao LH., Cui XZ., Yuan HJ., Liang B., Zheng LL., Liu YX., Luo MJ., Tan JH.. Restraint stress inhibits mouse implantation: temporal window and the involvement of HB-EGF, estrogen and progesterone. PLoS One 2013;8e80472. 10.1371/journal.pone.0080472.Couto BP, Silva HR, Filho AG, da Silveira Neves SR, Ramos MG, Szmuchrowski LA, Barbosa MP. Acute effects of resistance training with local vibration. Int J Sports Med. 2013; 34: 814-9.
. Couto BP., Silva HR., Filho AG., da Silveira Neves SR., Ramos MG., Szmuchrowski LA., Barbosa MP.. Acute effects of resistance training with local vibration. Int J Sports Med 2013;34:814–9. 10.1055/s-0032-1331198. 23444091.Dittrich N, de Lucas RD, Maioral MF, Diefenthaeler F, Guglielmo LG. Continuous and intermittent running to exhaustion at maximal lactate steady state: neuromuscular, biochemical and 50. He SB, Tang WG, Tang WJ, Kao XL, Zhang CG, Wong XT. Exercise intervention may prevent depression. Int J Sports Med. 2012; 33: 525-30.
Dittrich N., de Lucas RD., Maioral MF., Diefenthaeler F., Guglielmo LG.. Continuous and intermittent running to exhaustion at maximal lactate steady state: neuromuscular, biochemical and endocrinal responses. J Sci Med Sport 2013;16:545–9. 10.1016/j.jsams.2012.12.001. 23391432.He SB, Tang WG, Tang WJ, Kao XL, Zhang CG, Wong XT. Exercise intervention may prevent depression. Int J Sports Med. 2012; 33: 525-30.
He SB., Tang WG., Tang WJ., Kao XL., Zhang CG., Wong XT.. Exercise intervention may prevent depression. Int J Sports Med 2012;33:525–30. 10.1055/s-0032-1306325. 22504906.
