 |
 |
- Search
| Phys Act Nutr > Volume 26(4); 2022 > Article |
|
Abstract
[Purpose]
Medical recommendations for balanced control of exercise, physical activity, and nutritional intake after breast cancer diagnosis remain unclear. Therefore, this review aims to summarize effective exercise methods and dietary opinions by reviewing clinical trial results.
[Methods]
We systematically reviewed studies that evaluated 1) the relationship between exercise methods and quality of life improvement in patients with breast cancer and 2) the recommendations for physical activity, exercise, nutrition, and potential ways to improve life after breast cancer. To conduct this literature review, we searched the PubMed database for articles published until October 1, 2022, using the terms “physical activity OR exercise,” “breast cancer,” and “nutrition.” After a primary review of the retrieved articles, we included clinical trials in this systematic review.
[Results]
We hypothesized that physical activity improves the quality of life after the onset of breast cancer, suggesting that a balanced approach to aerobic exercise and resistance exercise increases the efficacy of anticancer treatment. From a nutritional point of view, it is recommended that both physical activity and diet management are necessary for patients with breast cancer.
[Conclusion]
Customized exercise and diet can help with weight loss, the reduction of cancer-induced fatigue, the regulation of hormonal changes, the reduction of inflammatory factors, and the improvement of mental health and vitality. Understanding the integrated mechanisms of physical activity and nutritional balance will improve the quality of life of patients with breast cancer. Therefore, it is necessary to continuously advance exercise programs and develop an alimentary balance control program.
Breast cancer ranks first among malignancies in women worldwide (one out of every 25 women). The causes of breast cancer can be attributed to mutations in tumor suppressor factors as well as epigenetic variables in the pathogenesis of breast cancer. In most cases, radiation therapy, chemotherapy, anticancer endocrine therapy, and targeted therapy are administered as adjuvant therapies after primary surgery to prevent the recurrence of breast cancer.
Exercise and physical activity have the advantage of presenting various benefits. Evidence suggests that exercise is associated with a longer health span and delays the onset of around 40 chronic conditions or diseases [1]. Regular exercise changes biochemical and molecular features and improves the quality of life. Exercises can be broadly classified into two types based on their form. The first is aerobic exercise, which involves exercising in a state where the oxygen supply is smooth during exercise. Aerobic exercise can typically be defined as prolonged steady-state exercise performed for durations between four minutes and four hours [2]. During exercise, the skeletal muscles require a large amount of oxygen, and the supplied oxygen, carbohydrates, and fats are used as fuel. Therefore, it facilitates metabolism by continuously using the fats and carbohydrates stored in the body. Because of these effects, endurance exercise helps lower mortality, facilitates weight loss and reduces abdominal obesity, improves cardiorespiratory, musculoskeletal, functional, and mental health and well-being (anxiety, cognitive health, and sleep), and reduces cancer risk [3]. Strength training is the second type of exercise. Strength training requires strong muscle contractions and, like aerobic exercise, promotes carbohydrate and fat metabolism. Because of its high strength, it is more dependent on carbohydrate metabolism than fat metabolism and may improve mechanical efficiency, muscle coordination, and exercise mobilization patterns [4]. In addition, it has been reported to reduce various metabolic diseases by lowering skeletal disease and mortality and promoting metabolism.
Epidemiological studies on the relationship between physical activity and cancer progression in recent years have suggested a reduction in the risk of cancer and its associated mortality rate [5]. Therefore, it is suggested that, for the effective application of adjuvant therapy, treatments should be developed to improve the quality of life of the patients.
Exercise not only activates physiological activity in the body but also influences homeostasis [6]. Therefore, current knowledge requires multifaceted analyses from a metabolic perspective to understand whether exercise is synergistic with medical guidelines and adjuvant therapies in patients with breast cancer. Exercise programs and diet affect the development of specific body parts and have also been proven to have an impact on mental health. This review summarizes the results of clinical trials on breast cancer survivors. It also presents the latest knowledge on exercise methods and diet management and discusses ways to improve their clinical efficacy and quality of life.
This review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines [7].
The literature search for this systematic review was conducted using three databases (PubMed) from the start of the study until October 1, 2022. This search used the following keywords in free text and medical subject headings (MeSH): “physical activity,” “breast cancer,” and “nutrition.” After a primary review of the retrieved articles, we included clinical trials in this systematic review. For this review, limits were applied to include only human studies, those conducted in English, and those conducted in patients with breast cancer. The search results were exported from the database in the “*.enl” format so that they could be included in a specific library in the reference manager, Endnote version X8 (Thomson Reuters, New York, USA).
The titles and abstracts of all retrieved publications were imported into EndNote, and duplicate and triplicate studies were excluded. When the available information on the titles and abstracts was insufficient, the full-text articles were read. The inclusion criteria were: (1) interventional studies; (2) randomized controlled trials; (3) studies on individuals with breast cancer or physical activity; and (4) studies with quality of life data as outcomes. The exclusion criteria were: (1) papers that had not undergone peer reviews, such as monographs and conference proceedings; (2) articles written in languages other than English; (3) papers that did not provide original text; (4) papers not focusing on humans; and (5) studies conducted using qualitative research methods.
The titles and abstracts were assessed for full-text retrieval. The full-text articles were assessed by two independent reviewers (PMY and CNN) by considering the inclusion and exclusion criteria to allow for adequate data extraction. The following data was extracted: type of intervention, classification of participants, sample size, and primary results. The study selection process is illustrated in Figure 1. A total of 1041 papers were identified, of which 160 were clinical trials. Among them, 28 research papers on various exercise programs and nutritional treatments were analyzed for breast cancer survivors.
Patients with breast cancer inevitably receive adjuvant chemotherapy to reduce their risk of metastasis. These patients may have weakened immune function because of chemotherapy; therefore, it is reasonable to provide them with proper nutrition to enhance immunity. According to the cancer management guidelines, it is recommended to continuously manage weight and intervene when changes in abdominal fat threaten health. A diminished amount of food significantly regulates body weight, abdominal fat, and visceral fat in patients with breast cancer [8]. However, the ketogenic diet did not show any signs of weight change in patients with breast cancer over a short period of time [9]. As shown in Table 1, green tea consumption increased high-density lipoprotein cholesterol maintenance, glucose homeostasis in the blood, and weight loss. In addition, several clinical trials have shown that the Mediterranean diet applied to patients with breast cancer activates factors that maintain good health [10-14].
Frequent physical impairments occur in patients with breast cancer after surgery, including but not limited to frozen shoulder, impinging shoulder syndrome, and lymphedema [15]. Additionally, in these patients, physiological parameters such as body weight, insulin resistance, cholesterol, and bone mineral density are influenced by side effects during chemotherapy and adjuvant therapy [16]. Patients may also experience psychological depression or insomnia [17]. The purpose of this review was to analyze exercise and nutritional intake that can help in the initial stages of breast cancer. In addition, we compared and analyzed exercise and diet, which resulted in significant clinical test results in breast cancer survivors. Table 2 summarizes the effects of exercise on various clinical trial participants.
The effectiveness of aerobic exercise on patients with breast cancer has been confirmed in one study, primarily in those with premenopausal breast cancer [18]. In this study, 966 patients with breast cancer aged 18-30 years who were physically active (at least twice a week) or nonsmokers with a body mass index (BMI) of 18-40 kg/m2 and a menstrual cycle of 24-35 days were randomly assigned into two groups. The control group consisted of those who followed a normal lifestyle. Next, the exercise group performed aerobic exercise five times a week for 30 minutes on a treadmill, stepper, and elliptical machine based on the maximum heart rate for 16 weeks. In this study, lean mass increased and body fat decreased in the exercise group. In addition, the ratio of 2-hydroxyestrone to 16α-hydroxyestrone, a metabolite of estrogen, was significantly higher than that in the control group.
The effect of resistance exercise on patients with breast cancer was confirmed in three instances. First, the effects of resistance exercise on body composition, muscle strength, lipids, inflammation, and oxidative markers have been investigated in postmenopausal breast cancer survivors who were prescribed tamoxifen [19]. In this study, 14 patients with breast cancer were analyzed and compared before and after exercise. For the adaptation period to exercise, a 12-week general warm-up (3-5 minutes of light activity) was performed, which was followed by leg press, chest press, knee flexion, front full motion rowing machine, and sit-up exercises six times or more in three sets each. After 120 seconds of rest, three sets of 12 or more strength exercises, 60-90 seconds of rest, and 20 repetitions of three sets of 45 seconds of rest were repeated three times a week. As a result, fat mass decreased and free fat mass increased. In addition, triglyceride and total cholesterol levels decreased after exercise, while high-density lipoprotein cholesterol increased. In addition, during the resistance exercise period, interleukin (IL)-6 levels increased and the activities of IL-17, thiobarbituric acid reactive substances, superoxide dismutase, and catalase decreased. Although resistance exercise improved the health indicators of exercise survivors who received tamoxifen, the positive effect of resistance exercise was not maintained when exercise was stopped. Resistance exercise has been studied for its immunological and anti-inflammatory properties in breast cancer survivors [20]. Thirty-nine patients with breast cancer were randomly divided into control and resistance exercise groups. Resistance exercises were performed for 16 weeks, and leg extensions, leg curls or Romanian deadlifts, lat pulldowns, machine bench presses, seated rows, back extensions, prone deadlifts, barbell squats, holds, or sit-ups were performed for 60 minutes per week. This procedure was repeated thrice. In the group subjected to resistance exercise, the expression of tumor necrosis factor-α and natural killer cells in the blood was significantly reduced compared with that in the control group. However, there was no change in the serum levels of IL-6, IL-10, or C-reactive protein. Hagstrom et al. studied the association between fatigue, quality of life, and resistance exercise in breast cancer survivors [21]. Patients with breast cancer aged 18-70 years who did not have recurrent breast cancer were classified into control and resistance exercise groups. Resistance exercise was performed three times a week for 60 minutes for 16 weeks, and the exercise program was divided into two parts. In Program 1, leg extensions, leg curls or Romanian deadlifts, lat pulldowns, machine bench presses, seated rows, back extensions, prone deadlifts, holds, or sit-ups were performed for eight weeks. Subsequently, in Program 2, barbell squats, deadlifts, free-weight barbell bench presses, leg presses, barbell bent-over rows, and assisted chin-ups were performed for eight weeks. Furthermore, the perceptions of fatigue and quality of life improved in the resistant group.
The effects of resistance and aerobic exercises on shoulder function in women after breast cancer surgery and radiation therapy were evaluated by measuring improved upper extremity isometric strength using the Disabilities of the Arm, Shoulder, and Hand questionnaire and Penn Shoulder Scale scores [22]. Obese women with stage 0-3 breast cancer were randomized, with a mean age of 53.5 years. The 16-week exercise program consisted of vigorous aerobic and resistance exercises. The patients performed aerobic and resistance exercises (leg press, chest press, lunges, seated row, knee extension, triceps extension, knee flexion, and biceps curl) for 150 minutes. The shoulder’s active range of motion and external rotation, upper extremity isometric strength, Disabilities of the Arm, Shoulder, and Hand, and Penn Shoulder Scale scores improved. In this review, the results of seven studies that analyzed the effects of aerobic and resistance exercises in parallel were investigated. The effects of aerobic and resistance exercises on bone health in patients with breast cancer have been studied [23]. One hundred patients with stage 0-3 breast cancer who completed adjuvant treatment and were non-smokers and physically inactive individuals with BMI ≥25.0 kg/m2 and waist circumference ≥88 cm were included in this study. Aerobic exercises were performed for 150 minutes, and resistance exercises (leg press, chest press, lunges, seated row, leg extensions, and triceps) were performed two days a week for 16 weeks. Fatigue, depression, maximal oxygen consumption (VO2max), muscle strength, osteocalcin, and B-cell-specific activator protein were higher in the exercise group. Furthermore, it was confirmed that breast lymphedema that occurs after surgical treatment for breast cancer is reduced by exercise [24]. Patients aged ≥18 years who underwent breast cancer surgery, including extensive local resection and axillary surgery, were randomly assigned to either the control or exercise groups. The exercises included stretching, a low-intensity warm-up, and the use of free weights, stationary bicycles, treadmills, rowing machines, and cross-trainers. For resistance exercises, abdominal, chest, and back exercises were performed for one hour, three times a week, for 12 weeks. In the exercise group, extracellular fluid measurements were reduced, and no worsening of lymphedema was observed. These findings suggest that exercise may further reduce breast lymphedema symptoms. Travier et al. evaluated whether exercise reduces fatigue due to disease in patients with breast cancer. In a clinical study, 204 patients with breast cancer were randomized to either regular therapy or aerobic and resistance training groups [25]. The exercises were performed in the order of warm-up, aerobic and strength training, and cool-down, for one hour twice a week for 18 and 36 weeks. At week 18, cardiorespiratory fitness and various muscle strength test results were significantly higher than those in the control group, and there was no difference in peak oxygen uptake. At week 36, there were no significant differences in cardiorespiratory and muscle strength tests. The physical fatigue was not statistically significant. Therefore, exercise during the early stages of breast cancer treatment may have a positive effect on patients with breast cancer. The Framingham Risk Score (FRS) is a method for predicting the development of cardiovascular diseases. The effects of aerobic and resistance exercises on FRS in overweight patients with early-stage breast cancer were evaluated 26 . One hundred patients with stage 1-3 breast cancer within six months after initiation of treatment were randomly assigned to the control or exercise groups. Endurance training (treadmill, cycloergometer, elliptical machine) was performed for 20 minutes, and resistance training (chest press, seated row, leg press, leg extension, biceps curl, and triceps extension) was performed for 40 minutes, three times a week, for 16 weeks. The FRS was significantly lower in the exercised group than in the control group. The cardiorespiratory effects according to changes in maximum oxygen consumption after aerobic exercise were then tested [27]. The two groups were analyzed for the effects of aerobic exercise on prostate and breast cancer survivors. Eighty-seven prostate cancer survivors (age range: 47-80 years) and 72 breast cancer survivors (age range: 34-76 years) were randomly assigned to groups that performed either high-intensity or low-intensity exercise for eight weeks. The exercise group continued an average of 25 minutes of aerobic exercise, 25 minutes of resistance exercise, and 10 minutes of static stretching three days a week for approximately one hour for eight weeks. The low-intensity exercise was performed at 60% VO2peak, and high-intensity exercise was performed at 75% VO2peak. The cardiorespiratory efficiency significantly increased by 0.5 ml/kg/min, and 2.2 ml/kg/min in the low and high-intensity groups, respectively.
Mijwel et al. reported the effects of high-intensity interval training (HIIT) on pain sensitivity, cancer-related fatigue (CRF), and life expectancy in patients with breast cancer undergoing chemotherapy [28,29]. So, in their study, patients with breast cancer were treated with resistance training and HIIT (RT-HIIT) and aerobic training and HIIT (AT-HIIT). In the RT-HIIT group, resistance exercises (leg presses, biceps curls, squat jumps, triceps extensions, lunges, bench presses, sit-ups or Russian-weighted abdominal twists, shoulder presses, and prone-lying back extensions) and high-intensity interval aerobic exercises (cycle ergometer, elliptical ergometer, or treadmill) were performed. In the AT-HIIT group, moderate-intensity continuous aerobic exercises were performed for 20 minutes, and high-intensity interval exercises were performed for 16 weeks. The RT-HIIT and AT-HIIT enhanced the cardiopulmonary function and decreased the BMI in the control group, whereas handgrip strength and lower-limb muscle strength were significantly improved in the RT-HIIT group. The RT-HIIT group showed a higher pain threshold than both groups. By decreasing CRF, HIIT also improved the quality of life for patients with breast cancer.
Some types of balloon dancing are aerobic exercises. The effects of light exercise on the quality of life of cancer survivors and their partners have been investigated [30]. Three months after the completion of primary chemotherapy, patients were assigned to the control (16 pairs) and exercise (15 pairs) groups. In the exercise group, Foxtrot, Waltz, ChaCha, and East Coast Swing were taught five times a week for five minutes each. Physical activity had a positive effect on vitality and trust between partners.
Subsequently, we summarize the effects of diet and exercise on patients with breast cancer. A total of 10 interesting results from our analysis are provided below.
Mefferd et al. analyzed the bioactivity index of 85 breast cancer survivors aged ≥18 years who were diagnosed with stage 1-3A breast cancer, completed early treatment, and had a BMI of 25.0 kg/m² (overweight or obese) [31]. The patients were given an average of one hour per day of physical activity and muscle-strengthening exercise, two to three times a week, and the diet was aimed at consuming 500-1000 kcal less per day. After 16 weeks, the participants lost weight and showed significant differences in BMI, fat percentage, body fat, leg fat, and waist and hip circumferences. Travier et al. investigated diet and physical activity for weight and nutritional patterns in women who survived breast cancer (n = 112) with a BMI ≥ 25 kg/m2 or greater [32]. The patients had a daily intake of 1200 to 1500 kcal/day, and they performed a 75-minute physical activity session biweekly: 10 minutes of warm-up, 25 minutes of aerobic exercise, 25 minutes of strength training using mats, stability balls, and resistance bands, and 10 minutes of stretching and rest. The patients showed significant weight loss and reductions in BMI, body fat mass, and waist circumference. Total energy, fat, saturated fat, and carbohydrate intake were significantly reduced, while the quality of life and CRF were significantly increased.
Buckland et al. studied 37 overweight and obese patients with early-stage breast cancer, aged 18-75 years, with a BMI of ≥25 kg/m2. Increased intake of fiber-rich foods and biweekly 75-minute sessions of physical activity (10 minutes of warm-up, 25 minutes of aerobic exercise up to 70% intensity on static bicycles, 25 minutes of strength training using mats, stability balls, and resistance bands, and 10 minutes of stretching and rest) were performed for 12 weeks [33]. The results showed that dietary carotenoid intake significantly increased, but plasma carotenoid levels did not. They also observed that the proportions of saturated fatty acids, n-6 polyunsaturated fatty acids, and monounsaturated fatty acids increased significantly. Karen et al. studied 38 overweight and obese patients with breast cancer who exercised and consumed a low-calorie diet for 13 weeks [34]. As a result, weight loss and a low-calorie diet reduced the waist circumference of the patient and increased vitality. Montagnese et al. in their study administered a Mediterranean diet and physical activity through a pedometer for 12 months to 227 breast cancer survivors with a mean age of 52.3 ± 9.3 years and normal weight [35]. The results showed that health status, social function, well-being, and sexual function improved, while fatigue, nausea and vomiting, shortness of breath, constipation, financial problems, systemic treatment side effects, and breast symptoms decreased. In another study conducted by Naderi et al., 30 breast cancer survivors with a mean age of 47.90 ± 7.95 years, a height of 160.93 ± 6.12 cm, and a weight of 72.62 ± 11.72 kg were treated with yoga and vitamin D for 12 weeks [36]. The participants were prescribed 4000 IU of vitamin D per day and instructed to perform Hatha yoga twice a week for 12 weeks. As a result, they showed increased grip strength, quality of life, and IL-10 levels and decreased tumor necrosis factor-α and IL-6 levels.
In a study by Carayol et al., 14 patients with early-stage non-metastatic breast cancer with an average age of 52 ± 10 years were treated with diet and exercise for 26 weeks [37]. These results suggest that the recommended regimen for improving the quality of life of patients is moderate-intensity mixed aerobic and resistance training sessions three times a week, along with a diet of 30-35% lipids, 50-55% carbohydrates, and 10-15% proteins. In a study by Lu et al., 104 breast cancer survivors with a mean age of 54.12 ± 10.25 years who received systemic adjuvant endocrine therapy for the first time were treated with diet and exercise for 10 weeks [38]. Based on these results, depression was resolved, and participants showed improved quality of sleep, fatigue due to cancer, and quality of life. In a study by Saxton et al., 85 overweight women with breast cancer were treated with individually tailored low-calorie therapy and exercise for 24 weeks [39]. The exercise that was optimized for this result included 30 minutes of aerobic exercise (age-predicted maximum heart rate: 65-85%). In addition, dietary intake was suggested to be 600 kcal per day less than the calculated daily energy requirement. With this method, depressive symptoms were reduced and the regulation of the hypothalamic-pituitary-adrenal axis was normalized.
Breast cancer is the most common invasive cancer among women in developed countries; however, its survival rate is increasing with advances in medical technology [40]. The longer the treatment method and duration of modern medicine, the higher the cancer survival rate, and the greater the economic burden on patients with cancer [41]. Cancer survivors are not just trying to survive; they are trying to achieve higher levels of quality of life [42]. In several reports, adjuvant therapies after surgery have been attempted to improve and maintain the quality of life of the patients [43]. Among the adjunctive prescriptions for a sustainable quality of life, physical activity and nutrition are key [43]. Although physical activity and nutritional balance are only optional, non-essential measures for patients with breast cancer, they now have the potential to become essential guidelines for breast cancer treatment [38]. In this review, we discuss the importance of physical activity and nutrition in improving the quality of life of patients with breast cancer by systematically reviewing and establishing the effects of physical activity and nutrition prescribed for these patients. The above analysis shows the importance of the Mediterranean diet in patients with breast cancer (Table 1). Nutritional management can help improve psychological stability and quality of life. However, maintaining homeostasis through temporary dietary changes improves the quality of life for the patients. Instead of a diet that expects pharmacological interpretation or physiological changes, it is necessary to transfer knowledge to patients for personal management. Our review also suggests the need to develop the capacity to continue learning and performing personal healthcare, which is important to the patient.
Previous studies have shown that the causes of breast cancer are closely related to hormones [44]. Some studies have reported that menopausal hormone changes in women cause a decrease in metabolism and an increase in various diseases, such as osteoporosis [45]. In addition, decreased physical activity, muscle mass, and metabolic rate increase obesity rates [46]. Furthermore, obesity is not only a key risk factor for breast cancer mortality in postmenopausal women but also contributes to the pathogenesis of breast cancer [46]. A recent report on breast cancer progression found that obese women in Korea (BMI, >25 kg/m2) had higher breast cancer severity than normal-weight women (BMI, 18-25 kg/m2) [47]. Being overweight or obese showed a high correlation with breast cancer [49]. Long-term obesity is known to cause various complications, including cancer, due to hormonal interactions resulting from an imbalance in endocrine factors, such as inflammatory cytokines [49]. Therefore, this review considers that the disease risk factors of patients should be focused on improving exercise protocols and nutritional management. The results of this analysis (Table 2) suggest that exercise methods increase the bioactivity index and control the risk of breast cancer based on the type and intensity of exercise. For breast cancer survivors, one study found that exercise intensity was effective over a long period since the patients performed adaptive physical activity at more than moderate intensity over the long term, which was consistent with their vital signs [26]. Moreover, moderate-intensity exercise reduces fatigue and increases activity in patients with breast cancer [50]. Interestingly, the results of one clinical trial showed that the synergistic effect of exercise and a nutritional diet could improve the quality of life of patients with breast cancer. In this regard, the study revealed a synergistic effect when exercising together while consuming a low-calorie or Mediterranean diet (Table 3). Moreover, the results are consistent with our argument that nutritional balance is involved in improving physical activity. Previous studies have suggested that the higher incidence of breast cancer is due to differences in diet and physical activity, and there is strong evidence to support the hypothesis that some features of the Western diet may increase the risk of breast cancer.
Clinical trials in breast cancer survivors have provided evidence for the importance of exercise and nutritional balance [31-39]. To the best of our knowledge, the long-term follow-up of patients after breast cancer surgery consists of the results and expectations shown in Figure 2. If adjuvant therapy is not administered after surgery, the quality of life falls below average and rapidly deteriorates. In contrast, applying adequate exercise and a controlled nutritional balance improves the quality of life and is expected to increase survival. Therefore, we also suggest that physical activity based on nutritional balance can be essentially a process to improve quality of life. Furthermore, we suggest that exercise and nutrition in patients with breast cancer are promising essential adjuvant therapies for breast cancer. Nevertheless, this review article has some limitations. The objective of this article was to review the existing literature and explore the relationship between physical activity and nutrition, as both may contribute to a greater duration and better quality of life in patients with breast cancer. The identified research indicates that physical activity is beneficial for survivor duration and quality; however, there are differences in the intensity of physical activity and nutritional composition. It is clear that additional research is necessary, particularly because of the variance of these findings between survival period, age group, type of physical activity, and nutrition and the physiological reasons for the effects of physical activity and nutrition on quality of life.
In conclusion, the Mediterranean diet and moderate-intensity exercise have beneficial effects such as weight loss, reduction of CRF, hormonal changes, regulatory roles, reduced inflammatory factors, and increased mental health and vitality. Therefore, physical activity and nutritional balance can improve the quality of life of patients with breast cancer. Future research should focus on developing patient-specific exercise programs and applying them to food balance programs.
Figure 1.
PRISMA Flowchart.
The PRISMA flow diagram for the systematic review detailing the database searches, the number of abstracts screened, and the full texts retrieved.
PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
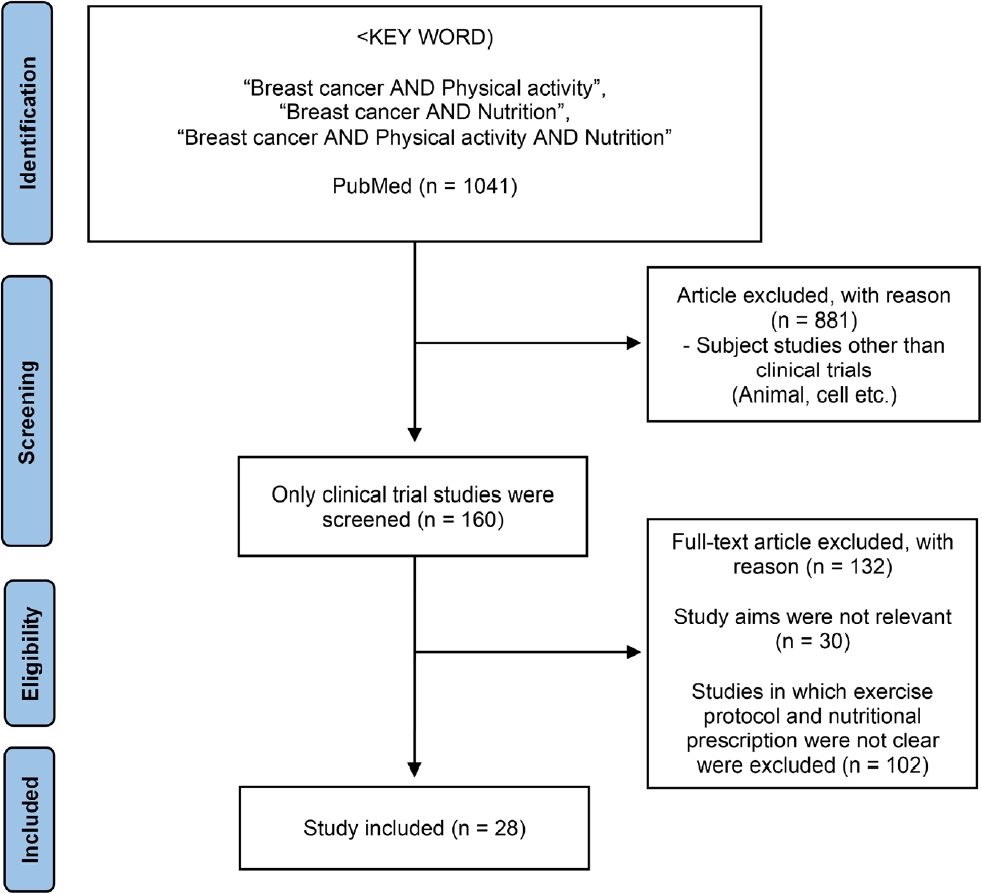
Figure 2.
Effects of physical activity and nutrition on quality of life and survival rate prediction in patients with breast cancer.

Table 1.
Effects of food and diet on physiological activity in patients with breast cancer.
| Diet / Food | Decreased factors | Increased factors | References |
|---|---|---|---|
| Mediterranean diet | HDL-cholesterol, body weight, body fat mass, n-6 fatty acid, waist circumference, fasting glucose, insulin resistance | Vitamin C levels, glucose polyunsaturated fatty acids n-9 Fatty acid | [10, 11, 12] |
| Green tea | Body weight | HDL, glucose homeostasis | [13] |
| Vitamin D | LDL-C | Muscle mass | [14] |
Table 2.
Effects of exercise on physiological activity in patients with breast cancer.
| Types of exercise | Exercise period | Clinical trial participants | Efficacy | References |
|---|---|---|---|---|
| Aerobic training | 5 times a week for 16 weeks | Patients with premenopausal breast cancer (n = 966) | Increased of estrogen metabolites (2-OHE1/16α-OHE1) and lean body mass | [18] |
| Decreased the fat mass and body fat | ||||
| Resistance training | 3 time a week for 12 weeks | Patients with postmenopausal breast cancer (n = 14) | Decreased the fat mass, triglycerides, total cholesterol, IL-6, IL-17, TBARS, SOD activity, and catalase activity | [19] |
| Increased free-fat mass and HDL-cholesterol | ||||
| 3 times a week for 16 weeks | Patients with breast cancer (n = 39) | Decreased the NK T-cell expression of TNF-α and NK cell expression of TNF-α | [20] | |
| 3 times a week for 16 weeks | Patients with breast cancer (n = 39) | Improved fatigue and quality of life | [21] | |
| Aerobic and resistance training | 3 times a week for 16 months | Patients with breast cancer (n = 100) | Increased in shoulder active range of motion | [22] |
| 2 times a week for 16 months | Patients with breast cancer (n = 100) | Improved fatigue, depression, muscular strength, osteocalcin, and BSAP | [23] | |
| For 12 weeks | Patients with breast lymph- oedema (n = 88) | No exacerbations of lymphoedema | [24] | |
| 2 times a week for 18 weeks | Patients with breast cancer (n = 204) | Positive effects on physical fatigue, cardiorespiratory function, and muscle strength | [25] | |
| 2 times a week for 16 months | Patients with obese breast cancer (n = 100) | Decreased Framingham Risk Score, systolic blood pressure, LDL-cholesterol, diabetes presence | [26] | |
| High-intensity exercise (VO2 peak: 75-80%) | Maintaining exercise habits | [27] | ||
| Improved quality of life | ||||
| High-Intensity interval training | For 16 weeks | Patients with breast cancer (n = 206) | Improved muscle strength and reduced pain sensitivity | [28] |
| Prevented the decline in cardiorespiratory fitness | ||||
| For 16 weeks | Patients with breast cancer (n = 206) | Decreased cancer-related fatigue and pain sensitivity | [29] | |
| Dance lessons | Ballroom dance for 12 weeks (at least 5 METs) | Patients with breast cancer (n = 31) | Improved physical activity, mental component of quality of life | [30] |
| Partners of patients with breast cancer (n = 31) |
HDL, high-density lipoprotein; LDL, low-density lipoprotein, MET, metabolic events; VO2, maximal oxygen consumption; BSAP, B-cell-specific activator protein; NK, natural killer; TNF, tumor necrosis factor; SOD, superoxide dismutase; TBARS, thiobarbituric acid reactive substances; 2-OHE1/16α-OHE1, 2-hydroxyestrone to 16α-hydroxyestrone.
Table 3.
A summary of the effects of combining exercise and diet on various clinical trial participants.
|
Condition |
Clinical trial participants | Efficacy | References | |
|---|---|---|---|---|
| Exercise | Diet | |||
| Physical activity (1 hour/day for 16 weeks) | Low-calorie diet (500-1000 kcal) | Overweight or obese breast cancer survivors (n = 85) | Decreased the weight, body mass index, percent fat, trunk fat, leg fat, waist, and hip circumference | [31] |
| Muscle-strengthening exercises (2-3/weeks for 16 weeks) | ||||
| Physical activity (75 minutes, biweekly for 12 weeks) | 1200-1500 kcal | Overweight or obese breast cancer survivors (n = 112) | Decreased the body mass, fat mass, and waist circumference | [32] |
| Increased quality of life and cardiorespiratory fitness | ||||
| Physical activity (75 minutes, biweekly for 12 weeks) | Low-calorie diet (vegetables, fish, and fiber-rich foods) | Overweight or obese breast cancer survivors (n = 112) | Decreased percentage of saturated fatty acids and n-6 polyunsaturated fatty acids, and ratio of long-chain n-6 to n-3 polyunsaturated fatty acids | [33] |
| Increased the monounsaturated fatty acids and total and long-chain n-3 polyunsaturated fatty acids | ||||
| Resistance and aerobic exercise | Low-calorie diet | Patients with breast cancer (n = 38) | Reduced the waist circumference | [34] |
| Improved the self-reported vitality scores | ||||
| Physical activity (for 12 months) | Mediterranean diet | Patients with breast cancer (n = 227) | Increased physical role and social functioning, body image, future perspective, and well-being | [35] |
| Reduced the fatigue, nausea and vomiting, dyspnea, and constipation | ||||
| Resistance and aerobic exercise (3 times per week for 26 weeks) | Lipids (30-35%), carbohydrates (50-55%), proteins (5%) | Patients with breast cancer (n = 14) | Positive changes in a range of psychological, physiological, and behavioral outcomes | [37] |
| Improved fatigue and quality of life | ||||
| Physical activity (3 times per week for 10 weeks) | High proteins, vitamins, fiber and high energy diet | Patients with breast cancer treated with systemic adjuvant endocrine drug therapy | Improved depression, sleep quality, cancer-induced fatigue, and life quality | [38] |
| Resistance and aerobic exercise (3 times per week for 24 weeks) | low-calorie diet | Patients with breast cancer (n = 85) | Reduced the depressive symptoms | [39] |
| Normalization of HPA axis regulation | ||||
REFERENCES
1. Ruegsegger GN, Booth FW. Health Benefits of Exercise. Cold Spring Harb Perspect Med 2018;8:a029694.



2. Shave R, Franco A. The physiology of endurance training. Churchill Livingstone. 2006. p. 61-84.
3. Bushman B; American College of Sports Medicine. ACSM’s Complete Guide to Fitness & Health(2E). Human Kinetics. 2017.
4. Suchomel TJ, Nimphius S, Bellon CR, Stone MH. The importance of muscular strength: training considerations. Sports Med 2018;48:765-85.



6. Travers G, Kippelen P, Trangmar SJ, González-Alonso J. Physiological function during exercise and environmental stress in humans-an integrative view of body systems and homeostasis. Cells 2022;11:383.



7. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev 2021;10:89.



8. Schapira DV, Clark RA, Wolff PA, Jarrett AR, Kumar NB, Aziz NM. Visceral obesity and breast cancer risk. Cancer 1994;74:632-9.


9. Khodabakhshi A, Seyfried TN, Kalamian M, Beheshti M, Davoodi SH. Does a ketogenic diet have beneficial effects on quality of life, physical activity or biomarkers in patients with breast cancer: a randomized controlled clinical trial. Nutr J 2020;22:87.


10. Pellegrini M, Ippolito M, Monge T, Violi R, Cappello P, Ferrocino I, Cocolin LS, De Francesco A, Bo S, Finocchiaro C. Gut microbiota composition after diet and probiotics in overweight breast cancer survivors: a randomized open-label pilot intervention trial. Nutrition 2020;74:110749.


11. Seethaler B, Basrai M, Vetter W, Lehnert K, Engel C, Siniatchkin M, Halle M, Kiechle M, Bischoff SC. Fatty acid profiles in erythrocyte membranes following the Mediterranean diet - data from a multicenter lifestyle intervention study in women with hereditary breast cancer (LIBRE). Clin Nutr 2020;39:2389-98.


12. Skouroliakou M, Grosomanidis D, Massara P, Kostara C, Papandreou P, Ntountaniotis D, Xepapadakis G. Serum antioxidant capacity, biochemical profile and body composition of breast cancer survivors in a randomized Mediterranean dietary intervention study. Eur J Nutr 2018;57:2133-45.



13. Stendell-Hollis NR, Thomson CA, Thompson PA, Bea JW, Cussler EC, Hakim IA. Green tea improves metabolic biomarkers, not weight or body composition: a pilot study in overweight breast cancer survivors. J Hum Nutr Diet 2010;23:590-600.



14. Kazemian E, Amouzegar A, Akbari ME, Moradi N, Gharibzadeh S, Jamshidi-Naeini Y, Khademolmele M, As’habi A, Davoodi SH. Vitamin D receptor gene polymorphisms affecting changes in visceral fat, waist circumference and lipid profile in breast cancer survivors supplemented with vitamin D3. Lipids Health Dis 2019;18:161.




15. Ebaugh D, Spinelli B, Schmitz KH. Shoulder impairments and their association with symptomatic rotator cuff disease in breast cancer survivors. Med Hypotheses 2011;77:481-7.


16. Yee LD, Mortimer JE, Natarajan R, Dietze EC, Seewaldt VL. Metabolic health, insulin, and breast cancer: why oncologists should care about insulin. Front Endocrinol (Lausanne) 2020;11:58.



17. Cho OH, Hwang KH. Association between sleep quality, anxiety and depression among Korean breast cancer survivors. Nurs Open 2021;8:1030-7.



18. Smith AJ, Phipps WR, Thomas W, Schmitz KH, Kurzer MS. The effects of aerobic exercise on estrogen metabolism in healthy premenopausal women. Cancer Epidemiol Biomarkers Prev 2013;22:756-64.




19. de Jesus Leite MAF, Mariano IM, Dechichi JGC, Giolo JS, Gonçalves ÁC, Puga GM. Exercise training and detraining effects on body composition, muscle strength and lipid, inflammatory and oxidative markers in breast cancer survivors under tamoxifen treatment. Life Sci 2021;284:119924.


20. Hagstrom AD, Marshall PW, Lonsdale C, Papalia S, Cheema BS, Toben C, Baune BT, Fiatarone Singh MA, Green S. The effect of resistance training on markers of immune function and inflammation in previously sedentary women recovering from breast cancer: a randomized controlled trial. Breast Cancer Res Treat 2016;155:471-82.



21. Hagstrom AD, Marshall PW, Lonsdale C, Cheema BS, Fiatarone Singh MA, Green S. Resistance training improves fatigue and quality of life in previously sedentary breast cancer survivors: a randomised controlled trial. Eur J Cancer Care 2016;25:784-94.

22. Sweeney FC, Demark-Wahnefried W, Courneya KS, Sami N, Lee K, Tripathy D, Yamada K, Buchanan TA, Spicer DV, Bernstein L, Mortimer JE, Dieli-Conwright CM. Aerobic and resistance exercise improves shoulder function in women who are overweight or obese and have breast cancer: a randomized controlled trial. Phys Ther 2019;99:1334-45.




23. Dieli-Conwright CM, Courneya KS, Demark-Wahnefried W, Sami N, Lee K, Sweeney FC, Stewart C, Buchanan TA, Spicer D, Tripathy D, Bernstein L, Mortimer JE. Aerobic and resistance exercise improves physical fitness, bone health, and quality of life in overweight and obese breast cancer survivors: a randomized controlled trial. Breast Cancer Res 2018;20:124.




24. Kilbreath SL, Ward LC, Davis GM, Degnim AC, Hackett DA, Skinner TL, Black D. Reduction of breast lymphoedema secondary to breast cancer: a randomised controlled exercise trial. Breast Cancer Res Treat 2020;184:459-67.



25. Travier N, Velthuis MJ, Steins Bisschop CN, van den Buijs B, Monninkhof EM, Backx F, Los M, Erdkamp F, Bloemendal HJ, Rodenhuis C, de Roos MA, Verhaar M, ten Bokkel Huinink D, van der Wall E, Peeters PH, May AM. Effects of an 18-week exercise programme started early during breast cancer treatment: a randomised controlled trial. BMC Med 2015;13:121.




26. Lee K, Tripathy D, Demark-Wahnefried W, Courneya KS, Sami N, Bernstein L, Spicer D, Buchanan TA, Mortimer JE, Dieli-Conwright CM. Effect of aerobic and resistance exercise intervention on cardiovascular disease risk in women with early-stage breast cancer: a randomized clinical trial. JAMA Oncol 2019;5:710-4.



27. Martin EA, Battaglini CL, Hands B, Naumann F. Higher-intensity exercise results in more sustainable improvements for VO2peak for breast and prostate cancer survivors. Oncol Nurs Forum 2015;42:241-9.


28. Mijwel S, Backman M, Bolam KA, Olofsson E, Norrbom J, Bergh J, Sundberg CJ, Wengström Y, Rundqvist H. Highly favorable physiological responses to concurrent resistance and high-intensity interval training during chemotherapy: the OptiTrain breast cancer trial. Breast Cancer Res Treat 2018;169:93-103.




29. Mijwel S, Backman M, Bolam KA, Jervaeus A, Sundberg CJ, Margolin S, Browall M, Rundqvist H, Wengström Y. Adding high-intensity interval training to conventional training modalities: optimizing health-related outcomes during chemotherapy for breast cancer: the OptiTrain randomized controlled trial. Breast Cancer Res Treat 2018;168:79-93.



30. Pisu M, Demark-Wahnefried W, Kenzik KM, Oster RA, Lin CP, Manne S, Alvarez R, Martin MY. A dance intervention for cancer survivors and their partners (RHYTHM). J Cancer Surviv 2017;11:350-9.




31. Mefferd K, Nichols JF, Pakiz B, Rock CL. A cognitive behavioral therapy intervention to promote weight loss improves body composition and blood lipid profiles among overweight breast cancer survivors. Breast Cancer Res Treat 2007;104:145-52.



32. Travier N, Fonseca-Nunes A, Javierre C, Guillamo E, Arribas L, Peiró I, Buckland G, Moreno F, Urruticoechea A, Oviedo GR, Roca A, Hurtós L, Ortega V, Muñoz M, Garrigós L, Cirauqui B, Del Barco S, Arcusa A, Seguí MA, Borràs JM, Gonzalez CA, Agudo A. Effect of a diet and physical activity intervention on body weight and nutritional patterns in overweight and obese breast cancer survivors. Med Oncol 2014 31:783. .



33. Buckland G, Travier N, Arribas L, Del Barco S, Pernas S, Zamora E, Bellet M, Cirauqui B, Margelí M, Muñoz M, Tusquets I, Arcusa A, Javierre C, Moreno F, Valverde Y, Jansen E, Chajès V, Castro C, Agudo A. Changes in dietary intake, plasma carotenoids and erythrocyte membrane fatty acids in breast cancer survivors after a lifestyle intervention: results from a single-arm trial. J Hum Nutr Diet 2019;32:468-79.



34. Basen-Engquist KM, Raber M, Carmack CL, Arun B, Brewster AM, Fingeret M, Schembre SM, Harrison C, Perkins HY, Li Y, Song J, Chen M, Murray JL. Feasibility and efficacy of a weight gain prevention intervention for breast cancer patients receiving neoadjuvant chemotherapy: a randomized controlled pilot study. Support Care Cancer 2020;28:5821-32.




35. Montagnese C, Porciello G, Vitale S, Palumbo E, Crispo A, Grimaldi M, Calabrese I, Pica R, Prete M, Falzone L, Libra M, Cubisino S, Poletto L, Martinuzzo V, Coluccia S, Esindi N, Nocerino F, Minopoli A, Grilli B, Fiorillo PC, Cuomo M, Cavalcanti E, Thomas G, Cianniello D, Pinto M, De Laurentiis M, Pacilio C, Rinaldo M, D’Aiuto M, Serraino D, Massarut S, Caggiari L, Evangelista C, Steffan A, Catalano F, Banna GL, Scandurra G, Ferraù F, Rossello R, Antonelli G, Guerra G, Farina A, Messina F, Riccardi G, Gatti D, Jenkins DJA, Celentano E, Botti G, Augustin LSA. Quality of life in women diagnosed with breast cancer after a 12-month treatment of lifestyle modifications. Nutrients 2020;13:136.



36. Naderi M, Kordestani H, Sahebi Z, Khedmati Zare V, Amani-Shalamzari S, Kaviani M, Wiskemann J, Molanouri Shamsi M. Serum and gene expression profile of cytokines following combination of yoga training and vitamin D supplementation in breast cancer survivors: a randomized controlled trial. BMC Womens Health 2022;22:90.




37. Carayol M, Ninot G, Senesse P, Bleuse JP, Gourgou S, Sancho-Garnier H, Sari C, Romieu I, Romieu G, Jacot W. Short- and long-term impact of adapted physical activity and diet counseling during adjuvant breast cancer therapy: the “APAD1” randomized controlled trial. BMC Cancer 2019;19:737.




38. Lu L, Chen X, Lu P, Wu J, Chen Y, Ren T, Li Y, Zhong X. Analysis of the effect of exercise combined with diet intervention on postoperative quality of life of breast cancer patients. Comput Math Methods Med 2022 2022:4072832. .




39. Tilli TM. Precision medicine: technological impact into breast cancer diagnosis, treatment and decision making. J Pers Med 2021;11:1348.



40. Smith GL, Lopez-Olivo MA, Advani PG, Ning MS, Geng Y, Giordano SH, Volk RJ. Financial burdens of cancer treatment: a systematic review of risk factors and outcomes. J Natl Compr Canc Netw 2019;17:1184-92.



41. Park J, Rodriguez JL, O’Brien KM, Nichols HB, Hodgson ME, Weinberg CR, Sandler DP. Health-related quality of life outcomes among breast cancer survivors. Cancer 2021;127:1114-25.



42. Wu DM, Wang S, Wen X, Han XR, Wang YJ, Shen M, Fan SH, Zhang ZF, Zhuang J, Shan Q, Li MQ, Hu B, Sun CH, Lu J, Zheng YL. Survival benefit of three different therapies in postoperative patients with advanced gastric cancer: a network meta-analysis. Front Pharmacol 2018;9:929.



43. Marquez DX, Aguiñaga S, Vásquez PM, Conroy DE, Erickson KI, Hillman C, Stillman CM, Ballard RM, Sheppard BB, Petruzzello SJ, King AC, Powell KE. A systematic review of physical activity and quality of life and well-being. Transl Behav Med 2020;10:1098-109.




45. Mili N, Paschou SA, Goulis DG, Dimopoulos MA, Lambrinoudaki I, Psaltopoulou T. Obesity, metabolic syndrome, and cancer: pathophysiological and therapeutic associations. Endocrine 2021;74:478-97.



46. Picon-Ruiz M, Morata-Tarifa C, Valle-Goffin JJ, Friedman ER, Slingerland JM. Obesity and adverse breast cancer risk and outcome: mechanistic insights and strategies for intervention. CA Cancer J Clin 2017;67:378-97.




47. Park IS, Kim SI, Han Y, Yoo J, Seol A, Jo H, Lee J, Wang W, Han K, Song YS. Risk of female-specific cancers according to obesity and menopausal status in 2•7 million Korean women: similar trends between Korean and western women. Lancet Reg Health West Pac 2021;11:100146.



48. Núñez-Ruiz A, Sánchez-Brena F, López-Pacheco C, Acevedo-Domínguez NA, Soldevila G. Obesity modulates the immune macroenvironment associated with breast cancer development. PLoS One 2022;17:e0266827.









