Resistance training inhibits the elevation of skeletal muscle derived-BDNF level concomitant with improvement of muscle strength in zucker diabetic rat
Article information
Abstract
[Purpose]
In the present study, we investigated the effects of 8 weeks of progressive resistance training on the level of skeletal muscle derived BDNF as well as glucose intolerance in Zucker diabetic rats.
[Methods]
Six week-old male Zucker diabetic fatty (ZDF) and Zucker lean control (ZLC) rats were randomly divided into 3 groups: sedentary ZLC (ZLC-Con), sedentary ZDF (ZDF-Con), and exercised ZDF (ZDF-Ex). Progressive resistance training using a ladder and tail weights was performed for 8 weeks (3 days/week).
[Results]
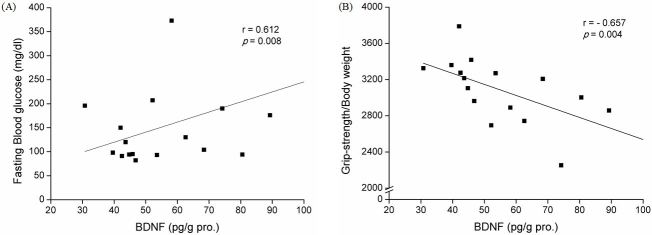
After 8 weeks of resistance training, substantial reduction in body weight was observed in ZDF-Ex compared to ZDF-Con. Though the skeletal muscle volume did not change, grip strength grip strength was significantly higher in ZDF-Ex compared to ZDF-Con. In the soleus, the level of BDNF was increased in ZDF-Con, but was significantly decreased (p<0.05) in ZDF-Ex, showing a training effect. Moreover, we found that there was a negative correlation (r=-0.657; p=0.004) between grip strength and BDNF level whereas there was a positive correlation (r=0.612; p=0.008) between plasma glucose level and BDNF level in skeletal muscle.
[Conclusion]
Based upon our results, we demonstrated that resistance training inhibited the elevation of skeletal muscle derived-BDNF expression concomitant with the improvement of muscle strength in zucker diabetic rats. In addition, muscle-derived BDNF might be a potential mediator for the preventive effect of resistance training on the progress of type 2 diabetes.
INTRODUCTION
Regular physical exercise is considered a cornerstone of therapeutic and preventive intervention for patients with type 2 diabetes mellitus (T2DM), and the importance of physical exercise is underscored by the costs and side effects that accompany pharmacological intervention. Recently, resistance training was reported to be effective for T2DM and may provide the additional benefit of preventing the musculoskeletal dysfunction associated with T2DM [1-4]. Resistance exercise induces a range of changes that facilitate muscle hypertrophy and whole body insulin action. Also, a robust finding in both human and rodent muscle in response to resistance exercise is an increase in the abundance of the glucose transporter GLUT4 isoform mRNA and protein [5]. Additionally, resistance exercise appears to have an impact on HbA1C that is significantly more than the effect of performing no exercise at all and similarly to aerobic exercise in T2DM patients [6].
Neurotrophins are well known to regulate several neuronal processes primarily through Trk receptor tyrosine kinases. The mammalian family of neurotrophins consists of nerve growth factor (NGF), neurotrophin-3 (NT-3), neurotrophin-4/5 (NT-4/5), and brain-derived neurotrophic factor (BDNF). Among these neurotrophins, BDNF and its receptor TrkB are the most widely and abundantly expressed in the brain [7]. Some studies suggest that BDNF might be involved in peripheral metabolism. Wisse and Schwartz (2003) reported that BDNF has been identified as a key modulator of the hypothalamic pathway that controls body composition and energy homeostasis [8]. Also, BDNF is thought to be a regulator of metabolism in skeletal muscles [9] and an enhancer of glucose utilization in diabetic skeletal muscles [10].
However, recent studies show that BDNF is also expressed in non-neurogenic tissues, including skeletal muscles. Given that BDNF can be expressed in skeletal muscles, animal studies had shown that BDNF mRNA increases in skeletal muscle in response to contraction [11,12]. BDNF mRNA and protein expression were increased in human skeletal muscles after exercise, however, skeletal muscle-derived BDNF appeared not to be released into the circulation [9]. Additionally, BDNF increased the phosphorylation of AMP-activated protein kinase (AMPK) and ACCβ and also enhanced fat oxidation. Based on recent knowledge, BDNF appears to be a myokine that acts in an autocrine or paracrine fashion with strong effects on peripheral metabolism, including fat oxidation, with a subsequent effect on the size of adipose tissue [13].
Although the important role of BDNF in peripheral metabolism including skeletal muscle is well known, the changes of BDNF expression in diabetic skeletal muscle with severe glucose tolerance have not been fully established. Moreover, considering the role of BDNF in skeletal muscle, it is essential to study the effect of progressive resistance training on BDNF expression with the improvement of muscle strength in diabetic skeletal muscle. In the present study, we investigated the effects of 8 weeks of progressive resistance training on the expression of skeletal muscle derived BDNF and its association with muscle strength in zucker diabetic rats.
METHODS
Experimental animals and experimental design
Male and female ZDF (fa/+) were purchased from Genetic Models (Indianapolis, ME) and mated with each other. They were housed in a conventional state under adequate temperature (23℃) and humidity (60%) control with a 12-h light/12-h dark cycle, and allowed free access to food and water. Purina 5008 rodent diets (7.5% fat) were provided as recommended by Genetic Models Co. (Purina, St. Louis, MO). Eighteen male lean (Zucker lean control, ZLC, +/+) and diabetic (Zucker diabetic fatty, ZDF, fa/fa) Zucker rats (age, 5 week old) were separated into 3 groups, namely lean control (ZLC-Con, n=6), diabetic control (ZDF-Con, n=6), and diabetic exercise-trained groups (ZDF-Ex, n=6).
Training began a week later. At 6- (pre-exercise) and 14 weeks (post-exercise) of age, all animals were evaluated for body weight (Mettler instrument AG CH-8606, Switzerland), grip strength (Bioseb, France), and fasting plasma glucose level (Roche Diagnostics LTD., Mannheim, Germany). After 8 weeks, the rats were sent for small-animal PET/CT evaluation and sacrificed 2 days after the PET imaging for skeletal muscle analysis. The procedures for handling and caring for the animals adhered to guidelines that are in compliance with the current international laws and policies (NIH Guide for the Care and Use of Laboratory Animals, NIH Publication No. 85-23, 1985, revised 1996), and the protocol was approved by the Institutional Animal Care and Use Committee (IACUC) of Seoul National University (SNU-131007-1). All of the experiments were conducted to minimize the number of animals used and the suffering caused by the procedures used in the present study.
Progressive resistance exercise training
The rats in the exercise-trained group (ZDF-Ex) were trained to climb a 1-m vertical (85 degree incline) ladder with weights secured to their tails. In their first week, the rats were familiarized with climbing up to the top cage with and without weight on their tails. Training sessions, from the second week, were commenced with intensity at 50% of each rat’s body weight; the weight in a conical tube was attached to the tail with a plastic belt and tape. Rats began climbing from the bottom of the ladder and were forced to climb to the top. When they reached the top, 2 min of rest was given and the next trial followed. Subsequent trials were performed from the bottom, and 20 g was added to the prior weight at every trial. If a rat was able to climb 10 times with the increasing weights, the training session was considered complete. In the event of failure to climb with the increased weight, the rat was forced to complete 10 trials with the last successful weight, with no further weight increase attempts.
Grip strength test
Grip strength was tested using a GripStrength Meter adapted with dual grip bars connected to two separate strain gauges to allow separate measurements of force with the two forepaws simultaneously. On each trial, the rat was held around the abdomen and lowered at an angle perpendicular to the bars until it griped the two bars, one with each forepaw, with its rear paws standing on the inclined surface of the apparatus. The rat was then pulled gently by the base of the tail, in a rearward direction, away from the bars. Rats naturally cling to the bars until they can no longer resist the pull, and then let go. The applied force at the point at which the rat released its grip for each paw was recorded by two separate strain gauges connected to a digital readout. Grip strength was tested in sessions of five trials, with approximately 1 min between each trial. Mean measurements for each rat were used.
Tissue collection and BDNF protein analysis
Rats were anesthetized by IP injection of Zoletil Zoletil 50 (10 mg/kg, i.p.; Vibac Laboratories, Carros, France). Tissues were collected from the gastrocnemius (GAS), soleus (SOL), tibialis anterior (TA), and the extensor digitorum longus (EDL), frozen on ice and stored at -80℃ until used. Samples were weighed and then homogenized using a RIPA buffer. Samples were spun at 14,000 r.p.m. for 20 min at 4°C, and the total protein concentration of the supernatant was determined by a Bradford assay.
The skeletal muscle levels of BDNF were measured by enzyme-linked immunosorbent assays (ELISA), according to the specifications of the manufacturer (Rat BDNF ELISA Kit; CUSABIO BIOTEC CO., LTD., Newark, DE). The detection range for the assay was 1.56 pg/ml to 100 pg/ml. All samples were tested in duplicate to guarantee the precision of the results and were run within the range of the standard curve. The results were expressed as the concentration of BNDF (pg/ml) read from the standard curves.
Statistical analysis
Statistical analysis was performed using the Origin 8.0 and SPSS 18.0 software package. The differences between values were compared with two-way repeated measure analysis of variance (ANOVA) followed by Dunnett’s multiple comparisons to estimate the body weight, grip strength, and plasma glucose concentration. Data are presented as means±S.E.M. with significance set at P<0.05. Associations of BDNF level in SOL with fasting blood glucose and grip strength were calculated with Pearson’s correlation coefficient.
RESULTS
As shown in Table 1, ZDF-Con rats were markedly heavier at the end of training than their lean littermates. After the 8-week exercise program, body weight was less in ZDF-EX than in ZDF-Con. The grip strength performance in ZDF-Con was significantly less than that of ZLC-Con. However, exercise training significantly improved grip strength in ZDF-Ex animals (p<0.05) but not in ZLC-Ex animals (data not shown).
The plasma glucose concentration in ZDF-Con rats gradually increased to 210.33 mg/dl during the 8-week training program. Fasting plasma concentration in ZDF-Con rats was markedly higher than in ZLC-Con rats. After the last exercise session in the 8 week study period, the fasting plasma level of glucose was lower in ZDF-Ex than in ZDF-Con, although the mean value was still higher than that of the ZLC-Con rats (Table 1).
There was a primary effect from the 8 weeks of resistance training on BDNF level in SOL (F=10.53, p<0.01). In the soleus muscle, ZDF-Con animals showed 39.9% lower level of BDNF compared to ZLC-Con animals. BDNF expression in the soleus of ZDF-Ex rats was 40.1% lower following resistance training when compared to ZDF-Con rats (p<0.05, Fig. 1). Although there was no significant change of BDNF level in TA, GAS, and EDL between ZLC-Con and ZDF-Con, the elevation of skeletal muscle derived BDNF was inhibited in TA (p<0.05, 28.7% reduction) and EDL (p<0.05, 13.1% reduction) muscles following 8 weeks of resistance training (Fig. 1). There was a positive association between the levels of plasma glucose and the level of BDNF in the soleus muscle (n=17; r=0.612; p=0.008; Fig. 2A). There was also a negative association between the values of grip strength and the level of BDNF in the soleus muscle (n=17; r=-0.657; p=0.004; Fig. 2B).

Effect of 8 weeks of resistance exercise on BDNF level in various skeletal muscles. (A) SOL, (B) TA, (C) GAS, and (D) EDL. *p<0.05 compared with ZLC-Con group, and #p<0.05 compared with ZDF-Con group. Values are mean±S.E.M. for n=6 in each group.
DISCUSSION
In the present study, we investigated the effect of progressive resistance training on BDNF expression and its relation to muscle strength in ZDF rat skeletal muscle. The main findings of our study are that the body weight and fasting plasma glucose concentration were reduced following 8 weeks of resistance exercise training. Grip strength and muscle strength were also significantly improved in trained diabetic animals. In the SOL muscles of ZDF rats, elevated BDNF was significantly decreased and was negatively correlated with grip strength. To our knowledge, this is the first study to investigate the effect of resistance training on muscle-derived BDNF level with concomitant improvement of muscle strength in transgenic diabetic animals.
Our results showed greater body weight reduction in ZDF-Ex rats than in ZDF-Con. Based on our observations, we could not find any significant differences in food and water intake between the exercise group and the non-exercise group (data not shown). Additionally, there was no visible symptom of under-nutrition during the entire resistance training program. In previous reports, resistance training led to reductions in HbA1c and fasting insulin [14], and improvement of insulin sensitivity [15]. Thus, in our results, the significant changes in body weight and grip strength observed as well as the improvement of glucose intolerance indicate that the resistance training was adequately performed.
Recently, the number of studies demonstrating the effects of resistance training on glycemic control and insulin sensitivity in patients with insulin resistance has substantially increased. Resistance training has been found to be effective for managing T2DM patients and may provide additional benefits for preventing or limiting muscular dysfunction related with T2DM [1-4,16]. Additionally, resistance training increases the protein contents of GLUT4, insulin receptor, glycogen synthase (GS) and GS total activity [17]. In our previous animal study, the expression of GLUT4 protein was increased in skeletal muscles using the same protocol as the present study, with high-intensity progressive resistance training using a ladder and tail weights (our unpublished data). Also, in the present study, our resistance training protocol was suitable for the T2DM model considering the fact that muscle strength was significantly increased concomitant with an improvement of glucose intolerance in trained diabetic rats. Taken together, our results suggest that progressive resistance training has a preventive effect on the impairment of glucose metabolism and the maintenance of muscle quality in T2DM patients.
It has been suggested that older adults with T2DM tend to have greater muscle loss, worse muscle quality, and reduced upper and lower limb strength than their healthy, age-matched counterparts [18-23]. Muscle quality and strength gains followed by resistance training may result in greater participation in physical activity [24-26], with more effective mobility functions, and glycemic profile improvements in T2DM patients [27]. In the present study, muscle strength was significantly improved following 8 weeks of resistance training in diabetic animals. These results indicate that resistance training could provide beneficial effects for diabetic patients.
In a previous study, increased BDNF mRNA in the soleus muscles of diabetic rats compared to age-matched controls implied that the elevation of BDNF may act to protect the distal nerve from denervation in diabetic rats [28,29]. In the present study, similar to the previous study, an increase of BDNF level in the soleus muscle of ZDF-Con compared to ZLC-Con was observed and BDNF level was significantly decreased following resistance exercise in SOL, TA, and EDL muscles as shown in Fig. 1. At 15 weeks of age, ZDF rats showed peripheral neuropathy involving a decrease in motor/ sensory nerve conduction velocities, myelin thickness, axon diameter, and axon thickness compared with the age-matched ZLC rats [30]. Studies in diabetic rats show that the levels of BDNF mRNA in the soleus muscle were elevated beginning at 4 weeks with a maximal six-fold increase by 6 weeks [31]. In the follow-up study, the researchers demonstrated that the increase in BDNF mRNA in the ipsilateral soleus muscle was dependent upon muscle activity [32]. It was proposed that the elevation of BDNF mRNA in diabetic skeletal muscle was an endogenous protective and/or repair mechanism induced by denervation of the gastrocnemius muscle [29]. In addition, muscle fiber damage in the diabetic soleus muscle results in an upregulation of BDNF mRNA in muscle fibers with the activation of satellite cells. According to previous reports and our data, BDNF in skeletal muscles might be expressed as a compensatory neurotrophic factor against diabetic neuropathy or myopathy.
Peripheral neuropathy and myopathy are common complications in patients with diabetes and lead to reduced muscle strength [33,34] due to progressive muscular atrophy [35]. Previous studies have shown that aerobic exercise training can ameliorate autonomic nerve dysfunction and induce diabetic nerve regeneration [36] in diabetic rats [37-39] while providing beneficial effects in experimental diabetic peripheral neuropathy [40]. However, the effects of resistance exercise training on diabetic neuropathy including motor neuron denervation or renervation are barely known. In the present study, BDNF level in the soleus muscles was positively correlated with surrogate factors for diabetes (fasting blood glucose) and negatively correlated with surrogate factors for physical fitness (muscle strength) as shown in Fig. 2. These results demonstrated that resistance training applied to the T2DM model induced not only an increase of BDNF level in skeletal muscle, but also an improvement of major variables for T2DM management including body composition, muscle strength, and fasting glucose level. As skeletal muscle derived BDNF has been identified as a novel contraction-induced cytokine that may contribute to the multiple health benefits associated with exercise [13], exercise induced BDNF in skeletal muscles might be an important mediator to elucidate the preventive effects of exercise for patients with T2DM.
The limitations of the present study include the lack of neuropathy and myopathy evaluation in diabetic animals. In the present study, also, the collected skeletal muscles contain intramuscular nerve endings, Schwann cells, vascular endothelial cells and connective tissue, in addition to skeletal muscle fibers. As in situ hybridization was not performed to determine the localization of BDNF expression, we cannot conclude which cells contributed to the increased BDNF in response to resistance exercise in the skeletal muscles of diabetic rats. The previous studies using in situ hybridization have confirmed that BDNF was expressed in the muscle fibers of both humans [41] and rats [42], and in the Schwann cells surrounding motor neurons [43]. Our results reflected not only an alteration of BDNF level in skeletal muscles, but also a complicated interaction in other cell types within the skeletal muscle. Therefore, further study would be needed to elucidate the alteration of BDNF level derived from various cell types in diabetic skeletal muscles.
In conclusion, our data showed that 8 weeks of resistance exercise inhibited the elevation of BDNF level in SOL with concomitant improvement of muscle strength. Based on the current knowledge, including our own findings, muscle- derived BDNF might be a potential mediator for the preventive effect of resistance training on the progress of type 2 diabetes. In addition, further research is needed to elucidate the regulatory role of muscle-derived BDNF in type 2 diabetic skeletal muscles.
ACKNOWLEDGEMENTS
This work was supported by the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (MEST 2011-0030135, NRF-2013M3A9B6046417, and Korea Mouse Phenotyping Project NRF-2013M3A9D5072550) and the Ministry of Education (NRF-2014R1A1A2058645).