Comparison of systemic and peripheral responses during high-intensity interval exercise under voluntary hypoventilation vs. hypoxic conditions
Article information
Abstract
[Purpose]
This study aimed to determine the systemic and peripheral responses to high-intensity interval exercise (HIIE) with voluntary hypoventilation at low lung volume (VHL) or HIIE under hypoxic conditions.
[Methods]
Ten male participants completed a single session of HIIE (three sets of 6 × 8-s high-intensity pedaling at 170% of maximal oxygen uptake [VO2max]) under three different conditions: normoxia with normal breathing (NOR: 23 °C, 20.9% of fraction of inspired oxygen [FiO2]), hypoxia with normal breathing (HYP: 23 °C, 14.5% FiO2), and normoxia with VHL (VHL: 23 °C, 20.9% FiO2). A randomized crossover design was used. Power output, arterial oxygen saturation (SpO2), heart rate, and muscle oxygenation were monitored during the exercise and the 16-s recovery. Muscle blood flow (mBF) of the vastus lateralis was also evaluated.
[Results]
SpO2 during the exercise and the 16-s recovery in the VHL group was significantly lower than in that of the NOR group. However, this parameter in the VHL group was significantly higher than that of the HYP group (NOR: 94.9 ± 0.4%, HYP: 82.8 ± 1.2%, VHL: 90.4 ± 0.5%; p < 0.001). Muscle oxygen saturation was significantly lower in the HYP group than those in the VHL and NOR groups (NOR: 79.6 ± 17.4%, HYP: 65.5 ± 7.7%, VHL: 74.4 ± 7.8%; p = 0.024). No significant difference in this parameter was observed between the VHL and NOR groups (p > 0.05). Additionally, the exercise-induced increase in mBF did not differ significantly among three groups (p > 0.05).
[Conclusion]
HIIE-induced SpO2 decrease was smaller under hypoxic conditions than during VHL. Moreover, mBF was not enhanced by the addition of VHL during HIIE.
INTRODUCTION
Team sports athletes are required to engage in repeated sprints for several seconds, separated by incomplete rest periods [1,2], which is defined as “repeated sprint ability” (RSA). RSA is associated with both aerobic and anaerobic metabolisms1. During the early phase of exercise, energy is mainly supplied by the creatine phosphate and glycolytic systems [3]. In contrast, during the latter phase of exercise, the contribution of the energy supply from the aerobic system is markedly augmented [4]. Therefore, the development of aerobic and anaerobic capacities is important for to improve RSA.
Recent studies have reported that repeated sprint exercise under hypoxic conditions (RSH) is effective in RSA improvement [2,5,6]. Despite previous inconsistencies of the findings regarding the benefits of RSH [7], RSH performed at high-intensity is reportedly more effective than other forms of hypoxic training. Brocherie et al. [5] reported that 4-5 weeks of RSH improved RSA more than the same training under normoxic conditions. RSH has been suggested to improve RSA primarily through peripheral adaptation (e.g., increased blood perfusion and improved fast-twitch muscle fiber activity during exercise) rather than hematological adaptation [8]. However, RSH requires special facilities to produce a hypoxic environment. From a practical perspective, it could be useful to propose a strategy that allows hypoxic training without the need for special facilities. Voluntary hypoventilation at low lung volume (VHL) could represent such an alternative. The effects of VHL during repeated sprint exercises have recently been explored [9-13]. During repeated sprint exercises with VHL, the participants started each sprint with a normal exhalation, then held their breath until the end of the sprint, and finally performed a second exhalation to empty the lungs of any remaining air [11,12]. Although repeated sprint training with VHL further increased RSA compared to the same training with normal breathing [9,10,12], to the best of our knowledge, no studies have directly compared the physiological responses occurring under a single session of RSH with those of a repeated sprint exercise with VHL. During repeated sprint exercises with VHL, arterial oxygen saturation (SpO2) decreased to approximately 90% [10,11], which was comparable to that during RSH [14,15]. Additionally, repeated sprint training with VHL for 3 weeks increased the oxygen uptake (VO2) during RSA test [12], which resulted from an increase in cardiac output. Previous studies of the acute effects of VHL exercise have shown a higher VO2 during the recovery periods [11,16,17]. This phenomenon might result from an augmented stroke volume because of a “pump effect” caused by the large inspirations after breath holding [16]. Thus, higher O2 availability is paramount during the recovery periods of RSA. The augmented O2 availability improves phosphocreatine resynthesis, which is the main energy component during repeated sprints, and may play a major role in developing anaerobic performance after training with VHL [12]. Moreover, RSA improvement after training with VHL may be different from physiological adaptations following RSH. RSH increases blood perfusion in muscles [18]. In addition, a previous study reported that exercise-induced peripheral tissue responses (e.g., changes in muscle blood flow and muscle oxygenation) were higher under hypoxic conditions [19]. In contrast, repeated sprint training with VHL may not improve the muscle oxidative capacity after the training period. In a previous investigation of changes in muscle oxygenation after 3 weeks of repeated sprint training with VHL12, VHL did not improve blood perfusion. Despite these findings, muscle oxygenation during repeated sprint exercises with VHL remains incompletely understood.
Therefore, this study aimed to compare the systemic and peripheral responses occurring during a single session of repeated sprint exercise with VHL with those of the same exercise under moderate hypoxic conditions. Moreover, we attempted to use VHL during high-intensity interval exercise (HIIE), which differed from the repeated sprint exercise with maximal effort in previous studies [11]. We hypothesized that the exercise-induced reduction in SpO2 during HIIE would be comparable between the two conditions. However, transient changes in muscle metabolism (e.g., muscle deoxygenation and muscle blood flow [mBF]) are expected to be smaller during HIIE with VHL than during the same exercise under moderate hypoxia.
METHODS
Participants
Ten healthy physically active males, who practiced recreational physical activity at least 2 days per week, were enrolled in the study (age [mean ± standard error]: 21.8 ± 0.4 years, height: 168.6 ± 1.5 cm, weight: 65.0 ± 2.2 kg). All participants resided at the sea level and none had been exposed to a different altitude as a training intervention. Moreover, none of the participants had any experiences with hypoventilation training before the present study. All participants were provided with an experimental overview and the possible risks of the study were delineated. Written informed consent was obtained from all the patients. This study was approved by the Research Ethics Committee of Ritsumeikan University (BKC2020-079) and was conducted in accordance with the Declaration of Helsinki.
Experimental overview
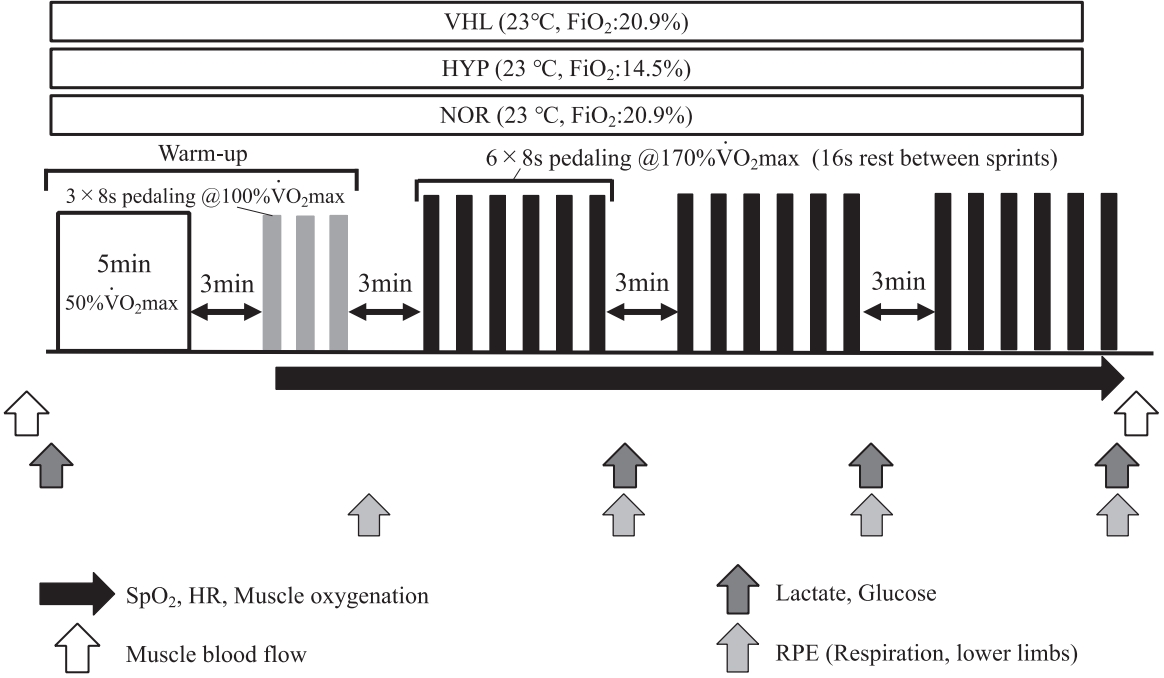
All the participants visited the laboratory five times during the experimental period. During the first visit, maximal oxygen uptake (VO2max) was assessed using a graded power test on a cycle ergometer with electromagnetic brake (Power Max VIII; Konami Corp., Tokyo, Japan). During the second visit, a familiarization session for HIIE with VHL was conducted. During the third to fifth visits, each participant performed the main trials, completing a single session of HIIE under three different conditions: normoxia with normal breathing (NOR: 23 °C, 20.9% of fraction of inspired oxygen [FiO2]), hypoxia with normal breathing (HYP: 23 °C, 14.5% FiO2), and normoxia with VHL (VHL: 23 °C, 20.9% FiO2). A randomized crossover design was used. Each trial was separated by at least two days. The trials were performed at the same time (between 13:00 and 15:00) of the day. All trials, including the familiarization session, were completed in a large (14.8 m2 ) normobaric hypoxic chamber (FCC-5000S; Fuji Medical Science, Chiba, Japan). The oxygen and carbon dioxide concentrations in the chamber were monitored continuously. Normobaric hypoxia was achieved under HYP conditions by insufflation of nitrogen into the chamber. SpO2 was monitored during the exercise and the 16-s recovery using a finger pulse oximeter (ATP-W03; Fukuda Denshi, Tokyo, Japan) placed on the tip of the left forefinger. Power output, heart rate (HR), and muscle oxygenation of the vastus lateralis muscle were monitored during the exercise and the 16-s recovery. Blood samples were collected to determine changes in blood glucose and lactate concentrations after each of the three exercise sets, and exercise-related sensations were evaluated simultaneously. The mBF of the vastus lateralis muscle was measured using venous occlusion and near-infrared spectroscopy (NIRS) immediately after exercise completion [19,20].
Exercise protocol
The HIIE was performed using a cycle ergometer with electromagnetic brake (Power Max VIII; Konami Corp.). The pedalling frequency was 100 rpm throughout the exercise period. Additionally, pedaling frequency was fixed using a metronome. The participants were warmed up before the main trials. The warm-up exercise included submaximal pedaling at 50% of VO2max and subsequent HIIE (3 × 8-s pedaling at 100% of VO2max). A 16-s recovery at 30% VO2max was provided between sprints. After the warm-up, the participants commenced the HIIE (three sets of 6 × 8-s pedaling at 170% of VO2max), with a 16-s recovery at 30% of VO2max between sprints and a 3-min passive rest period between sets (Figure 1). This exercise intensity (170% of VO2max) has been utilized in previous studies of HIIE [21]. A previous study using 170% of VO2max presented marked improvements in both anaerobic and aerobic capacities [21].
The participants underwent HIIE with VHL, except for the recovery period between sprints, which was performed with normal breathing. The procedure of HIIE or repeated sprint exercise with VHL has been described in detail previously [10-12]. Briefly, the participants were required to start each sprint with a first exhalation down to the functional residual capacity, then to hold their breath until the end of the 8-s sprint exercise, and finally to perform a second exhalation down to the residual volume to evacuate the carbon dioxide accumulated in the lungs [11,12,22]. In a familiarization session, SpO2 was monitored to confirm whether HIIE with VHL could successfully lower SpO2, as previously reported [10,11].
MEASUREMENTS
Maximal oxygen uptake
The VO2max was assessed at the first visit using a graded power test. Before the test, the participants performed a warm-up session of 5 minutes of pedaling at 100 W. The test began at 70 W, and the load was increased progressively in 30 W increments every 2 minutes until voluntary exhaustion (100 rpm). Respiratory gases were collected and analyzed using an automatic gas analyzer (AE-300S; Minato Medical Science Co., Ltd., Tokyo, Japan). The data were averaged every 30 s. HR was measured continuously during exercise using a wireless HR monitor (RCX5; Polar Electro, Tokyo, Japan). The rating of perceived exertion (RPE) was determined every 2 minutes using a 10-point scale [23].
Measurements during the main trial
During HIIE, the peak and mean power outputs were evaluated for each 8-s sprint under all conditions. SpO2 was recorded continuously during the 8-s exercise and the 16-s recovery (every second) with a pulse oximeter on the left forefinger (ATP-W03; Fukuda Denshi). HR was recorded continuously during the exercise and the 16-s recovery (every second) using a wireless HR monitor (RCX5; Polar Electro). SpO2 was presented as the minimum value during the exercise and the 16-s recovery. HR was presented as the average value for all sprints.
Muscle oxygenation variables (oxygenated hemoglobin [oxy-Hb], deoxygenated hemoglobin [deoxy-Hb], total hemoglobin [total-Hb], and tissue oxygen saturation [StO2]) were evaluated by NIRS (Hb14; ASTEM Co., Ltd., Kanagawa, Japan) [24] in the vastus lateralis muscle at a distance of 50% from the greater trochanter and lateral condyle of the femur. To determine muscle oxygenation variables, the NIRS probe was placed on the muscle at an inter-optode distance of 30 mm. All NIRS variables were measured before (during 1 minute of rest before warm-up under normoxic conditions with participants in a supine position) and during each 16-s recovery period (averaged values). During exercise with VHL, the maximum and minimum values of SpO2 were expected to appear for a few seconds following the end of the previous sprint [11]. Therefore, the data during the recovery periods were analyzed. All signals were recorded at a sampling frequency of 10 Hz. NIRS data during the exercise and the 16-s recovery under each condition were expressed as relative values to resting levels (before exercise).
To evaluate mBF, venous occlusion was performed on the right leg (most proximal part) using an automatic rapid cuff inflation system (E20; Hokanson, Inc., Bellevue, WA, USA) and a 10-cm wide cuff (SC10D; Hokanson, Inc.) with participants in the supine position. The mBF was evaluated before the warm-up and 40 seconds immediately after exercise completion. This procedure was used in a previous study [24]. Venous occlusion (70–100 mmHg) was applied for 20 seconds to restrict venous outflow. The mBF was calculated in milliliters per minute per 100 g of muscle tissue (mL/min/100 g) from the linear increase in total-Hb during venous occlusion (monitored using NIRS) [20,25-27].
Blood samples were collected from the fingertips before the warm-up (in each condition) and immediately after each set of exercises. Blood glucose and lactate concentrations were immediately measured using a glucose analyzer (Free Style; Nipro Corp., Osaka, Japan) and lactate analyzer (Lactate Pro; Arkray Inc., Kyoto, Japan), respectively.
Perceived breathing difficulty (RPEbreath) and lower limb discomfort (RPEleg) were rated immediately after each set using a 10-point scale [23].
Statistical analysis
All statistical analyses were performed using the SPSS software (ver. 27.0; IBM Corp., Armonk, NY, USA). All data were expressed as means ± standard errors. Two-way repeated-measures analysis of variance (ANOVA) was used to assess the interaction and main effects of condition and time. A one-way repeated-measures ANOVA was conducted to evaluate the main effect of the condition on the average power output, HR, and SpO2. Significant interactions and main effects were further analyzed using the Tukey-Kramer post-hoc test. Statistical significance was set at p < 0.05.
RESULTS
SpO2 and HR
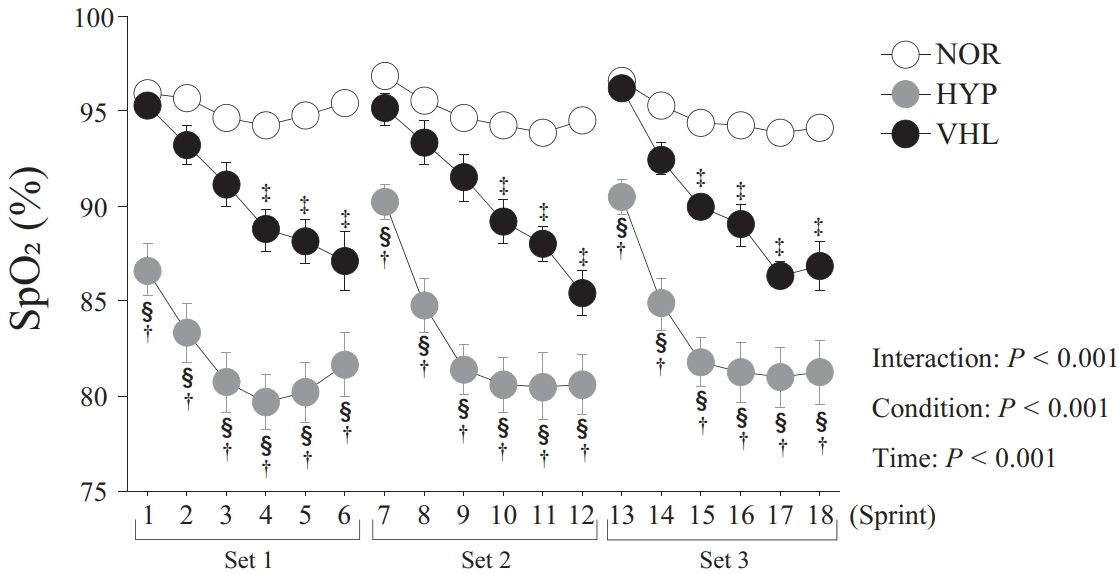
The average values of the minimum SpO2 during the exercise and the 16-s recovery were 94.9 ± 0.4% in the NOR group, 90.4 ± 0.5% in the VHL group, and 82.8 ± 1.2% in the HYP group. SpO2 in the VHL group was significantly lower than that in the NOR group (p < 0.001, Figure 2). However, this parameter in the VHL group was significantly higher than that in the HYP group (p < 0.001). The maximum and mean HR in the HYP group were significantly higher than those in the VHL and NOR groups (p = 0.005).
Power output
The peak power output did not differ significantly among three conditions (Table 1). VHL group presented a slightly (< 1.9%) but significantly lower mean power output than HYP and NOR groups (p = 0.043).
Blood variables
The blood lactate concentration increased significantly after exercise in all conditions (p < 0.001). Additionally, post-exercise blood lactate concentrations were significantly higher in the HYP group than those in the VHL and NOR groups (p = 0.001). The blood glucose concentration did not differ significantly among three conditions (p > 0.05, Table 2).
Muscle oxygenation
The oxy-Hb level decreased significantly during the 16-s recovery period in the HYP group (p < 0.001, Figure 3). While the oxy-Hb level in the HYP group was significantly lower than that of the NOR group (p = 0.032), there was no significant difference between the VHL and NOR groups (p > 0.05). The deoxy-Hb level increased significantly during the 16-s recovery period under all conditions (p < 0.001, Figure 3). However, no significant difference was observed among three conditions (p > 0.05). The total-Hb level increased significantly during the 16-s recovery period under all three conditions (p < 0.001, Figure 3). However, no significant difference was observed among three conditions (p > 0.05). StO2 decreased significantly during the 16-s recovery period under all conditions (p < 0.001, Figure 3). Furthermore, StO2 in the HYP group was significantly lower than those in the VHL and NOR groups (p = 0.024). However, there was no significant difference between this parameter in the VHL and NOR groups (p > 0.05).
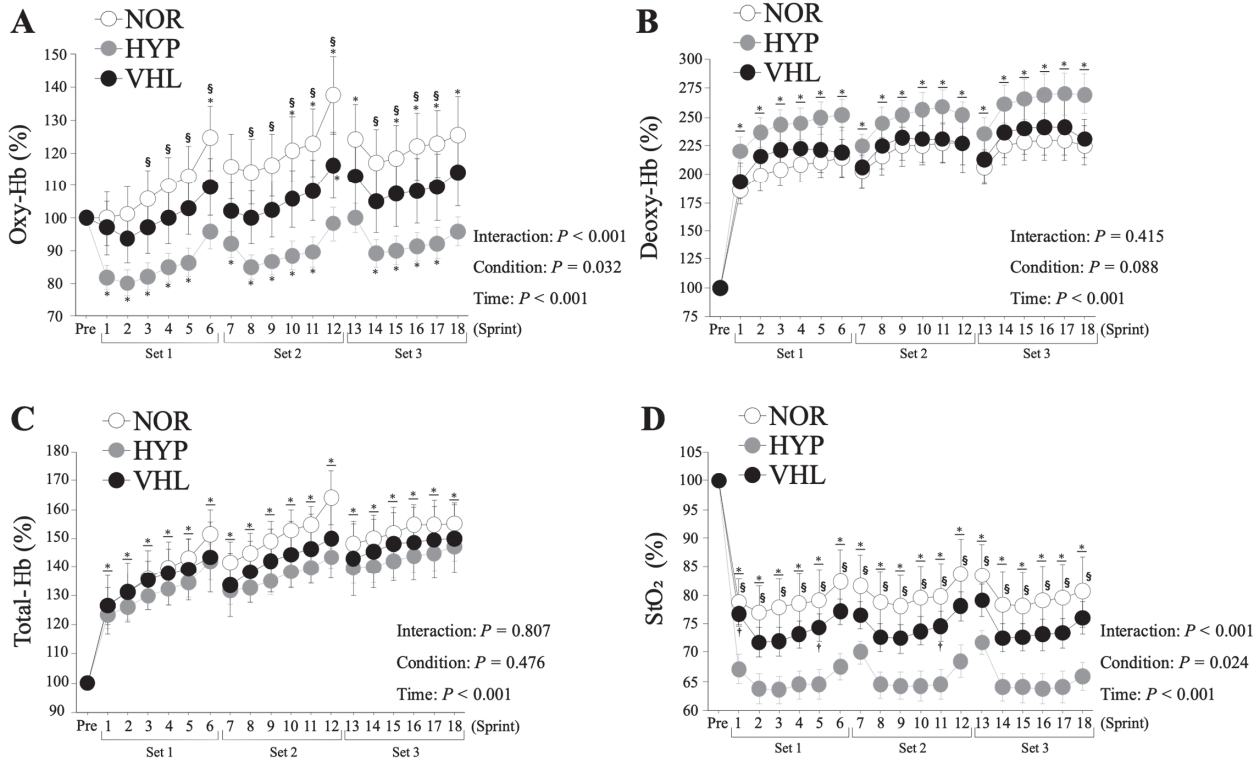
Changes in oxy-hemoglobin (A), deoxy-hemoglobin (B), total-hemoglobin (C), and tissue oxygen saturation (D) during the 16-s recovery between sprints. Values were presented as means ± standard errors. *p < 0.05, comparison with the pre-exercise condition (Pre). †p < 0.05, comparison between the VHL and HYP groups. §p < 0.05, comparison between the HYP and NOR groups.
Muscle blood flow
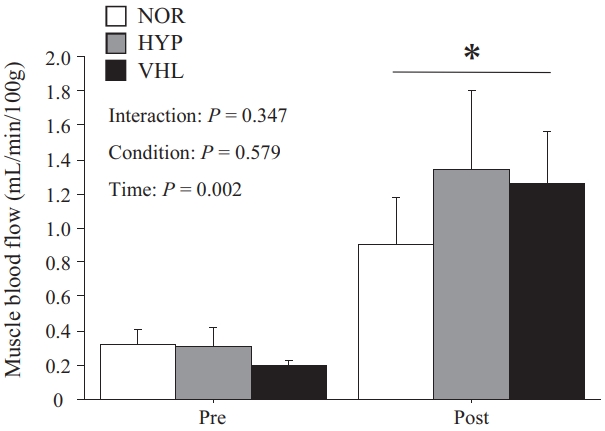
The mBF increased significantly immediately after exercise under all three conditions (p = 0.002, Figure 4). However, no significant difference was found among three conditions (p > 0.05).
Perceptual responses
RPEbreath and RPEleg increased significantly with exercise under all conditions (p < 0.001, Table 3). The average value of RPEbreath in the VHL group was significantly higher than those in the HYP and NOR groups (p < 0.001). In contrast, RPEleg in the HYP group was higher than that of the VHL group during exercise set 2 (p = 0.032).
DISCUSSION
This is the first study to compare the systemic and peripheral responses occurring in a single session of HIIE with VHL and those parameters of the same exercise under moderate hypoxia. Our main findings were as follows: (1) SpO2 was significantly lower under VHL condition than NOR condition, (2) SpO2 was significantly higher under VHL condition than HYP condition, (3) muscle oxygen saturation was significantly lower in the HYP condition than the VHL and NOR conditions, with no significant difference between the latter two, and (4) exercise-induced increases in mBF did not differ significantly among three conditions.
Systemic and peripheral variables
As expected, SpO2 during the exercise and the 16-s recovery in the VHL group was significantly lower than that of the NOR group. Additionally, the decrease in SpO2 under the VHL condition was comparable to that reported in previous studies [10,11]. Breath holding promotes hypoxia in the body. During exercise with VHL, CO2 output was inhibited, and CO2 accumulated in the body due to breath holding [28], leading to an increase in its partial pressure in the blood. Moreover, the increased partial pressure of CO2 shifted the oxygen dissociation curve to the right, which decreased the affinity of hemoglobin for oxygen, leading to a lower SpO2 (i.e., the Bohr effect). Although we were unable to determine the partial pressure of CO2, Woorons et al. [16] reported that exercise with VHL increased the partial pressure of CO2 compared with the same exercise using normal breathing. Thus, exercise with VHL would facilitate hypoxemia due to the augmented partial pressure of CO2, which may lower blood and muscle pH. Moreover, the exercise-induced decrease in SpO2 promoted compensatory vasodilatation by augmenting NO production [29]. Yamaguchi et al. [24] reported a higher muscle blood volume during repeated sprint exercises during hypoxia than during normoxia. Increased muscle blood volume augments the shear stress of vascular endothelial cells, leading to the enhanced production of vascular endothelial growth factor (VEGF) [30]. As VEGF promotes angiogenesis, these physiological responses may improve the oxygen supply to the working muscles and the clearance of metabolites during high-intensity exercise [31-33].
While the exercise-induced increase in blood lactate concentration was greater in the HYP group than in the VHL and NOR groups, no significant difference was found between the latter two. Several studies demonstrated that post-exercise blood lactate concentrations were higher in RSH compared to the same exercise under normoxia [33-35]. In addition, augmented blood lactate concentration after exercise with VHL (versus the same exercise with normal breathing) has been previously reported [28,36] with some inconsistencies [11]. Further investigation is required to compare the exercise-induced acid-base disturbance between repeated sprint exercise with VHL and the same exercise with normal breathing under an equivalent power output.
Muscle blood flow
The decrease in SpO2 during HIIE with VHL was unstable, and fluctuations in SpO2 have also been observed previously [37]. The breath holding during VHL only occurred during the exercise, not during the rest periods between sprints, creating an intermittent decrease in SpO2. Moreover, StO2 in the HYP group was significantly lower for those in the VHL and NOR groups. However, there was no significant difference between the VHL and NOR groups. In addition, the mBF did not differ significantly immediately after exercise among the three conditions. Therefore, VHL during HIIE did not affect oxidative metabolism in the working muscles. Similarly, Woorons et al. [17] reported that repeated sprint exercises with VHL did not affect muscle tissue oxygenation. Therefore, VHL during repeated sprint exercises had relatively minor impact on local blood flow and oxygen utilization in working muscles. On the other hand, Woorons et al. [38] reported the greater increase in deoxy-Hb during repeated sprint exercise with VHL. Since RSH has been reported to improve oxygen utilization in the fast-twitch muscle fibers [8], the same physiological responses might occur during repeated sprints under hypoxic conditions. Woorons et al. [38] used the VHL method with end-expiratory breath holding held up to the breaking point during exercise and reported the lowest level of SpO2 among all VHL studies. Therefore, the greater fall in muscle oxygenation might result from severe arterial desaturation. Breath holding for as long as possible (i.e., up to the breaking point) may be a strategy to trigger a decrease in SpO2 and muscle deoxygenation.
Perceptual variables
The RPEbreath in the VHL group was significantly higher than those in the HYP and NOR groups. Previous studies have suggested that different mechanisms were involved in the improved performance of repeated sprint training with VHL in comparison to RSH11. RSH did not increase oxygen uptake during exercise compared to the same exercise under normoxia [39,40]. However, repeated sprint exercises with VHL were associated with higher oxygen uptake during rest periods between sprints than the same exercise using normal breathing [11]. Since the RPEbreath was higher under VHL condition in the present study, metabolic stress on the respiratory muscles, rather than the vastus lateralis muscle, might increase by utilizing VHL.
The present study had several limitations. We used HIIE (equivalent to 170% of VO2max) rather than repeated sprinting exercises ( exercise with maximal effort) because we thought that HIIE at fixed exercise intensity with VHL would be applicable, as compared to exercise with maximal effort. However, the mean power output was slightly, but significantly, lower in the VHL condition than in the other two conditions, which may affect the present outcomes. Therefore, a similar comparison is required using repeated sprint exercises with VHL. Moreover, since the decrease in SpO2 was lower in exercise with VHL than in hypoxic conditions (14.5% FiO2), a comparison to moderate hypoxia around 16.4% FiO2 (a simulated altitude of 2,000 m) may be more appropriate.
In conclusion, HIIE-induced SpO2 decrease was smaller under hypoxic conditions than during VHL. Moreover, mBF was not enhanced by the addition of VHL during repeated sprint exercises.
Acknowledgements
We appreciate the participation of all subjects in the present study. This study was funded by a research grant from Ritsumeikan University.