Rosae multiflorae fructus regulates the lipogenesis in high-fat diet-induced NAFLD mice model
Article information
Abstract
[Purpose]
Exercise helps modify the lipid profile in the body, partly through its impact on sterol regulatory element binding protein-1 (SREBP-1) and peroxisome proliferator-activated receptor-γ (PPAR-γ). Individual differences in response to exercise and genetic variations may influence the response to PA. Therefore, this study explored Rosae multiflorae fructus (RMF) as a supplement candidate that improves exercise capacity and controls non-alcoholic fatty liver disease (NAFLD) by suppressing lipogenesis and controlling lipid peroxidation.
[Methods]
RMF is a natural herbal medicine used in Dongui Bogam. RMF has antioxidant, anti-inflammatory, and anti-allergic effects. However, the effects of RMF on NAFLD have not yet been investigated. In this study, we examined the effects of RMF in a mouse model of high-fat diet-induced NAFLD. Mouse livers were isolated and analyzed using H&E staining and immunohistochemistry.
[Results]
RMF downregulated lipid peroxidation markers, such as CYP2E1, in the livers of mice with high-fat diet-induced NAFLD. Additionally, the RMF significantly reduced the lipid accumulation-related protein expression of CD36, SREBP-1, and PPAR-γ.
[Conclusion]
RMF exerts anti-lipid peroxidation and anti-lipogenic effects in a high-fat diet-induced NAFLD mouse model.
INTRODUCTION
Obesity is a medical condition characterized by the excessive accumulation of body fat, which may impair health [1]. Obesity can have mental and psychological effects, including depression, anxiety, and low self-esteem [2]. Obesity can cause various diseases, including chronic type 2 diabetes, high blood pressure, heart disease, certain cancers, strokes and respiratory problems [3]. Non-alcoholic fatty liver disease (NAFLD), caused by obesity, is the most common chronic liver disease, with an average of 20% of cases reported worldwide [4]. As modern society becomes more westernized, the number of patients with NAFLD is rapidly increasing due to eating habits [5]. Non-alcoholic fatty liver disease is a condition in which excessive neutral fat accumulates in the liver [6]. In a few cases, simple steatosis progresses to non-alcoholic steatohepatitis; however, the relationship between the two diseases has not been elucidated. However, non-alcoholic steatohepatitis shows faster histological progression than simple steatosis and has a higher probability of developing cirrhosis and liver disease-related mortality [7]. Therefore, one of the most important aspects of modern science is research on ways to suppress or treat obesity.
Numerous studies have established a strong relationship between physical activity and diminished obesity [8-10]. The molecular mechanisms underlying the relationship between exercise and lipid metabolism-related key factors like sterol regulatory element binding protein-1 (SREBP-1), peroxisome proliferator-activated receptor-γ (PPAR-γ), CD36, CYP2E1, and lipid metabolism [11]. Dietary changes combined with exercise effectively reduce the risk of developing metabolic diseases [12]. Furthermore, studies have reported that natural products, such as herbs, botanical extracts, and dietary supplements, have been studied for their potential effects on obesity-related factors [13]. In this study, we attempted to analyze Rosae multiflorae fructus (RMF), a representative natural product for treating circulatory diseases, as specified in Donguibogam, an important book of Korean medicine [14]. However, the role of RMF in liver disease remains unclear. Therefore, in this study, we explored the inhibitory effects of RMF on lipid synthesis in an NFALD model.
METHODS
Extraction of RMF
Rosae multiflorae fructus (RMF) extraction was performed using a slightly modified version of a previously described method [15]. Briefly, a crude extract was prepared using RMF (100 g) in 1,000 mL of sterile deionized water and heated at 100 °C for 3 h. The decoction product was concentrated and lyophilized by freeze-drying at −60 °C using a Dongbang VFDL03-50 freeze-dryer (Dongbang Hi-tech incorporation, Seongdong-gu, Seoul, Korea).
Animal care
Animal care was performed as previously described [15]. Briefly, the C57BL/6J mice were purchased from Orient Bio (Gyeonggi-do, South Korea). All animal procedures were approved by the Institutional Animal Care and Use Committee of Semyung University (approval number 19-10-01), and the experimental protocols complied with the NIH Guide for the Care and Use of Laboratory Animals. To create the NAFLD mouse model, the mice were fed a high-fat diet (60% fat, 20% carbohydrates, and 20% protein) for 24 weeks. Next, a pharmacological efficacy test was conducted for 8 weeks. The mice were randomized into two groups: the HF group, a high-fat diet with normal saline (HF), and the HF+RMF group, a high-fat diet combined with RMF (3 mg/kg/day).
Histological analysis and immunohistochemistry assay
To confirm the effects of RMF in the NAFLD mouse model, tissues were analyzed using a staining solution and specific antibodies. Immunohistochemistry was performed as previously described [16]. First, the mice were perfused and fixed in 10% formalin. Then, the liver specimens were prepared according to the routine procedures for cryosection. Sections were stained with Masson’s trichrome (M/T). Next, the other specimens were placed in 0.3% hydrogen peroxide for 3 min to block endogenous peroxidase activity and then blocked using 5% goat serum for 1 h. The tissues were subsequently incubated for 72 h at 4 °C in 3% goat serum containing the various primary antibodies. Antibodies to CD36 (SC-7309), SREBP-1 (SC-365513), and PPAR-γ (SC-7273) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The anti-CYP2E1 (ab28146) antibody was obtained from Abcam (Cambridge, UK). The tissues were subsequently washed three times using phosphate-buffered saline, and the Vectastain ABC Kit was used to induce avidin–biotin complex interactions, according to the manufacturer’s instructions. Immunostaining analysis was performed using a substrate solution of 0.05% DAB, and the slides were counterstained with hematoxylin. All the sections were evaluated under a light microscope (BX50; Olympus Co., Ltd., Hachioji-shi, Tokyo, Japan) at ×200 magnification.
Statistical analysis
Statistical data are expressed as mean ± standard error based on at least three independent experiments. All data were analyzed using GraphPad Prism software (Prism 4.00 Windows, GraphPad, San Diego, CA, USA), based on Student’s t-test for intergroup differences and one-way analysis of variance with Tukey’s test for multiple comparisons. Statistical significance was set at P <0.05 significant.
RESULTS
RMF decreased lipid accumulation in high-fat diet-induced mice
To investigate whether RMF can inhibit lipid accumulation in the liver tissue of HFD-fed mice, we performed histological analysis using M/T staining. As shown in Figure 1, numerous lipid droplets were observed in the liver tissue of HF mice compared to those in untreated (UN) mice. However, the administration of RMF to HF showed that RMF significantly downregulated the number of lipid droplet regions.
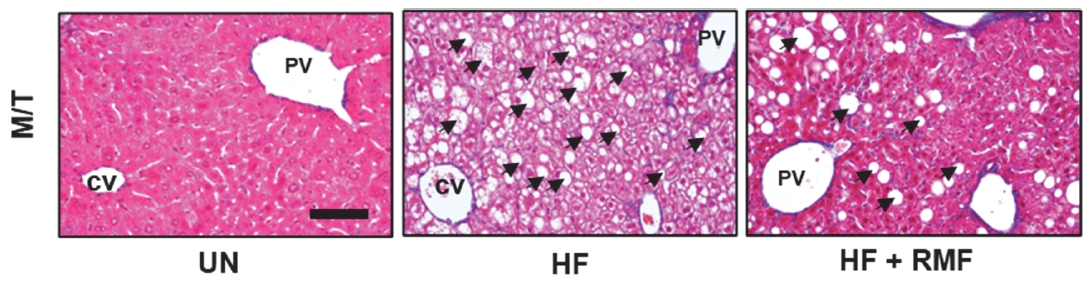
Effect of Rosae multiflorae fructus (RMF) on histological changes in liver tissues of high fat diet-induced obese mice. The mice were randomly divided such as untreated group, high fat diet (HF) group, high fat diet and treated with RMF (HF + RMF) group. The mice were sacrificed, and liver tissues were isolated. The liver tissues were stained with Masson’s trichrome and observed under the microscope. The block arrow indicates the lipid. CV, Central vein; PV, Portal vein. Bar size, 50 μm.
RMF reduces the expression of CYP2E1 in high-fat diet mice
To investigate whether RMF could regulate lipid peroxidation in mice, the expression of CYP2E1 was detected using immunohistochemistry. As shown in Figure 2A and B, the HF diet increased the intensity of CYP2E1 up to 6896.13 ± 27.55 % compared to that in the UN group. Whereas the HF+RMF group indicated the expression of CYP2E1 to be 375.91 ± 45.58 %.

Effect of Rosae multiflorae fructus (RMF) on CYP2E1 expression in liver tissues of high fat diet-induced obese mice. The micewere randomly divided such as untreated group, high fat diet (HF) group, high fat diet and treated with RMF (HF + RMF) group. The liver tissues were stained with anti-CYP2E1. The expression of CYP2E1 in tissues were detected using the microscope. *p < 0.05 vs HF group. The block arrow indicates the lipid. CV, Central vein; PV, Portal vein. Bar size, 50 μm.
RMF reduces the expression of CD36, SREBP-1, and PPAR-γ in high-fat diet mice
To examine the effect of RMF on liver lipid accumulation, mice were fed a high-fat diet or treated with RMF extract. CD36, SREBP-1, and PPAR-γ were determined using the specific antibodies. As shown in Figure 3A, HF significantly increased the intensity of CD36 to 636.07 ± 19.10 %, compared to that in the untreated group. In contrast, HF+RMF significantly decreased the CD36 to 341.0 ± 13.4 % compared to that in the HF group. In the case of PPAR-γ, HF significantly increased the intensity of PPAR-γ to 738.46 ± 50.29 %, compared to that in the untreated group. In contrast, HF+RMF significantly decreased the CD36 to 388.5 ± 43.6 % compared to that in the HF group (Figure 3B). Similarly, HF significantly increased the intensity of SREBP-1 to 554.02 ± 20.33 %, compared to that in the untreated group. In contrast, HF+RMF significantly decreased the CD36 to 292.9 ± 13.6 %, compared to that in the HF group (Figure 3C).

Effect of Rosae multiflorae fructus (RMF) on lipogenesis related proteins in high fat diet-induced obese mice. The mice were divided such as untreated group, high fat diet (HF) group, high fat diet and treated with RMF (HF + RMF) group. The liver tissues were stained with specific antibodies such as anti-CD36, anti-PPAR-γ and anti-SREBP-1. The expression of CD36, PPAR-γ and SREBP-1 in tissues were detected using the microscope. The block arrows indicate the lipid. *p < 0.05 vs HF group. CV, Central vein; PV, Portal vein; SREBP-1, sterol regulatory element binding protein 1; PPAR-γ, peroxisome proliferator-activated receptor- γ. Bar size, 50 μm
DISCUSSION
Rosa multiflora is divided into RMF and Rosa multiflora Thunb (RM). Several recent studies have shown that RM effectively improves sleep quality and acts as an antioxidant and anti-inflammatory agent [17,18]. In addition, RMF exhibits anti-atopic, anti-allergic, and anti-inflammatory effects similar to those of Donguibogam in Korean Oriental medicine [19,20]. However, studies on NFALD in the RMF and RM have not yet been conducted. To address these issues, we investigated the effects of HFD-induced NAFLD on the RMF.
NAFLD is associated with excessive neutral fat, contributing to steatohepatitis and cirrhosis in normal liver tissues [21]. This causes damage to normal tissues but is a dangerous disease because the patient does not feel pain as the lesion progresses. Toda S et al. suggested that herbs with superior anti-inflammatory effects could modulate peroxidation in an ischemic injury model [22,23]. Song et al. reported that RMF has strong antioxidant and anti-inflammatory activities [24]. Non-alcoholic fatty liver disease is accompanied by lipid peroxidation due to excessive fat synthesis, and the expression of CYP2E1 is promoted in the liver tissue [25]. We explored the inhibitory effect of RMF on the overexpression of CYP2E1 using noncytotoxic concentrations of RMF. Our results showed that CYP2E1 was overexpressed in the liver tissues of NAFLD animal models induced by a high-fat diet. In contrast, its expression was significantly decreased in the RMF group. Therefore, we suggest that RMF has strong antioxidant efficacy and can inhibit lipid peroxidation caused by NAFLD.
Numerous studies have shown a strong relationship between physical activity and obesity reduction [26,27]. Molecular mechanisms underlying the relationship between exercise and key factors such as SREBP-1, PPAR-γ, and lipid metabolism [28]. Several studies have shown that physical activity may help reduce the levels of SREBP-1, which regulates the genes responsible for fatty acid and triglyceride synthesis [30,31]. Physical activity and exercise may contribute to maintaining a healthy balance in lipid metabolism by reducing adipogenesis, which is regulated by SREBP-1 [32]. Exercise may affect the expression and activity of PPAR-γ [33]. Our results specifically implicate RE intake in regulating PPAR-γ and SREBP-1 expression in adipose tissue. Moreover, we observed that the expression of CD36, which promotes fatty acid transport in the liver, was inhibited by RMF. Therefore, we propose that RMF may be an effective herb for improving exercise capacity and dietary control.
In conclusion, we suggest that RE is a potential candidate for treating NAFLD and as a sports supplement because of its ability to significantly reduce lipogenesis and lipid peroxidation. The effect was observed to occur through modulation of SREBP-1, PPAR-γ, CD36, and CYP2E1. However, based on our data, we could not determine the synergistic effects of exercise and RMF intake. Further, in vivo experiments are required to confirm the effects of exercise protocols and RMF intake.
Acknowledgements
This paper was supported by the Semyung University Research Grant of 2022.