Endurance exercise training inhibits neointimal formation via enhancement of FOXOs expression in balloon-induced atherosclerosis rat model
Article information
Abstract
[Purpose]
This study investigated the effect of endurance exercise on neointimal formation, endothelial-dependant relaxation and FOXO expression in balloon-induced carotid arteries of rats.
[Methods]
Male SD(Sprague-Dawley) rats of 8 weeks ages were randomly divided into 3 groups; Sham-operated control (SO, n=10), Balloon-induced control (BIC, n=10), and Balloon-induced exercise (BIE, n=10). Endurance exercise training was performed on treadmill (18 m/min, 0% grade, 60 min/day, 5 days/week, 4 weeks).
[Results]
Body weight is significantly reduced in BIE compared with BIC. Neointiaml formation in BIC was significantly higher than SO, but it was significantly recovered in BIE compared with BIC. Endothelial-dependent relaxation in BIC was significantly lower than SO, but it was significantly recovered in BIE compared with BIC and expression of FOXO1 and FOXO3a also were significantly increased BIE compared with BIC.
[Conclusion]
These data suggest that endurance exercise inhibits neointimal formation and endothelial-dependent relaxation via FOXO expression in balloon-induce atherosclerosis rat model.
INTRODUCTION
One of characteristic markers of atherosclerosis is endothelial dysfunction. Oxidative stress to the vascular endothelium is a serious causative factor of vascular endothelial dysfunction [1-3].
Damage of vasculatures (endothelial cells and smooth muscle cells) that play an important role in vascular contraction and relaxation is caused by diverse signal transductions, which has been confirmed in medical experiments on living human atherosclerotic arteries [4].
Atherosclerosis is a chronic inflammatory disease of vessels which is known to be associated with blood lipids disorder. Numerous studies have reported that decrease of risk factors such as serum lipid, blood serum lipoprotein, hypertension, diabetes and hyper-sensitive C-reactive protein (hs-CRP) can delay development of atherosclerosis [5]. However, its molecular mechanism for the prevention of the atherosclerosis has not been studied widely.
As of now, two main mechanisms have been explain the underlying cause of atherosclerosis: lipid hypothesis and chronic endothelial injury hypothesis [5].
In chronic endothelial injury hypothesis, SIRT1 (Sirtuin1) is highly expressed in the endothelium, where it controls the angiogenic activity of endothelial cells. In other words, SIRT1 overexpression prevents aging of endothelial cells that induce oxidative stress, while SIRT1 inhibition increases aging of endothelial cells [6]. Inhibition of SIRT1 in vasculature decreases endothelial-dependent relaxation through dysfunction of eNOS (endothelial nitric oxide synthase) deacetylation [7-9]. Overexpression of SIRT1 in ApoE-knockout mice attenuated atherosclerosis [10].
Importantly, SIRT1 is known to deacetylate multiple targets in mammalian cells, including Forkhead box O1 and 3(FOXO1 and FOXO3) [11,12]. FOXO recently attracted attention as its association with reactive oxygen species (ROS) [13,14], apoptosis [15,16], glucose metabolism [17,18], and cardic myocyte size [19,20] became known.
It also has been reported that FOXO plays an important role in development of atherosclerosis [21,22]. FOXO has close relations with activation of phosphatidylinositol-3-kinase (PI3K)/AKT, shear stress and generation of ROS [23]. It is also known to inhibit migration of vascular smooth muscle cells and neointima formation [21,22,24]. However, role of exercise for these has not been reported yet.
Regular physical activity improves endothelial-dependent relaxation which is protective against eNOS-NO dysfunction in hypertension and atherosclerosis. Blood flow-derived vascular relaxation by exercise is reportedly caused by increase of shear stress [25].
Several studies have reported that regular exercise was very effective in inhibiting atherosclerosis [26,27]. Regular exercise has been found to fast recover damaged endothelioid cells and inhibit neointima formation which marks the beginning of atherosclerosis [28-30]. A recent study reported that increase of shear stress regulates the activation of SIRT-eNOS in endothelial cells [8]. However, it focused mainly on damage of endothelial cells caused by deactivation of SIRT1-eNOS in vascular cells (endothelioid cells and smooth muscle cells). Not a study has been done on regular physical activity that can increase FOXO activation in vascular tissue.
Therefore, this study investigated the role of endurance exercise on neointimal formation, endothelial-dependent relaxation and expression of FOXO proteins in an animal model of atherosclerosis.
METHODS
Animal care
6-week-old male SD (Sprague-Dawley, n = 30) rats provided by Science Lab Center Co., Ltd. were used in this study. A week was allowed for the animals to adapt to the new surroundings and then the rats were divided into a BI (balloon-injured-atherosclerosis group) and a SO (shamoperated control group). SO went through the same operation procedure as others but atherosclerosis was not induced in SO. Mice in BI were randomly divided into two groups: BIC (balloon-injured control group) and BIE (balloon-injured and exercise group), resulting in separate three groups: SO, BIC and BIE. The animals were kept in lab cages (30 cm × 20 cm) by each group in the same temperature (20-25℃), same humidity (50-60%) and same contrast (12 hour cycle). The rats were given sterile water and feed ad libitum. Management and experiment procedures for the animals used in this study observed ethics regulations of Animal Testing Ethics Committee of C University (CNU-00052).
Balloon angioplasty models
In order to induce atherosclerosis in rats, an anesthetic (a mixture of ketamine 80 mg/kg and xylazine 12 mg/kg) was injected in intraperitonealy, and then 2F forgaty Catheter (Edwards Lifesciences, Irvine, Calif) was inserted into common carotid artery (CCA) through an external carotid artery (ECA). The pressure gauge was used to inflate the catheter balloon 1.5 times greater than diameter of carotid artery and then 10 mm injury was induced by the withdrawal of inflated balloon catheter 5 times [31].
Endurance exercise protocol
The rats were exercised on a treadmill (motorized rodent treadmill) for 4 weeks in order to investigate the effects of endurance exercise. The rat began running on treadmill three days after their endothelioid cells were damaged. They ran at 10m/min with a 0% incline for 10 minutes on the first day and the speed and duration of the exercise were increased by 10 minutes and 2 m/min every day until the 4th day. From the 5th day to the end of the experiment, the rat ran at 18m/min for 60 minutes [28].
Endothelial-dependant relaxation
A 2~3mm blood vessel from an atherosclerotic carotid arteries was harvested from the rat and was attached to a force transducer (Adinstruments, USA), while bathed in a Krebs buffer solution (NaCl 100 mM, KCl 4.7 mM, CaCl2 1.9 mM, MgSO4 1.2 mM, K2HPO4 1.03 mM, NaHCO2 25 mM, pH 7.4) in order to see how much the vessel was contracted in response to the solution. The vessel was contracted with 0.3 uM L-phenylephrine hydrochloride (PE) and subsequently relaxed by a cumulative addition of acetylcholine chloride (Ach) to investigate endothelial-dependent relaxation, using Chartpro software (Adinstruments, USA) [32].
Hematoxylin & Eosin staining for measurement of neointimal formation
Balloon-injured rat carotid arteries were fixed in 4% formaldehyde and pafaffin-embedded. Serial cross sections (5 μm thick) of arteries were stained with hematoxylin and eosin (MHS-32, Sigma, USA). DP70 camera (Olympus, Tokyo) and TSView version 7 (Fuzhou Tucsen Image Technology, Japan) were used to measure size (um2) of intima, media and lumen to calculate the intima-media thickness and lumen diameter to compare the degree of neointimal formation [31].
Western blotting
The vessel tissue was homogenized in lysis buffer (20 mM Tris HCl, 0.5% NP-40, 250 mM NaCl, 3 mM EDTA, 3 mM EGTA, 2 mM DTT, 0.5 mM phenylmethylsulfonylfluoride, 2 mM b-glycerophosphate, 1 mM sodium vanadate, 1 lg/ml leupeptin and pH 7.5., Sigma, USA) and then centrifuged for 30 minutes at 14,000 rpm at 4 ℃ to remove the supernatant and quantify proteins, using BCA assay Kit Kit (Bio-rad, USA). 50 ug of proteins were subject to electrophoresis on 9% SDS-PAGE and then transferred to a PVDF membrane. Nonspecific reaction of the membrane was removed by blocking it for one hour at room temperature in 5% nonfat dry milk in TBS-T. FOXO1(Cell signaling, USA), FOXO3a (Cell signaling, USA) and Beta-actin (Sigma, USA) were incubated for 18 hours at 4°C in TBST with 5% nonfat dry milk. HRP-conjugated rabbit-antimouse IgG (Calbiochem, USA) was used for secondary antibodies. Blots were developed for visualization using an enhanced chemiluminescence detaction Kit (Thermo, USA) and ImageQuant software (Molecular dynamics, USA) was used to quantify the expression.
Statistical analysis
SPSS Statistics was used to calculate the descriptive statistics quantity from the results of this study and one-way ANOVA was used to verify each variable, while Duncan’s test was used for post hoc analysis. ɑ = 0.05 was classified to have statistical significance.
RESULTS
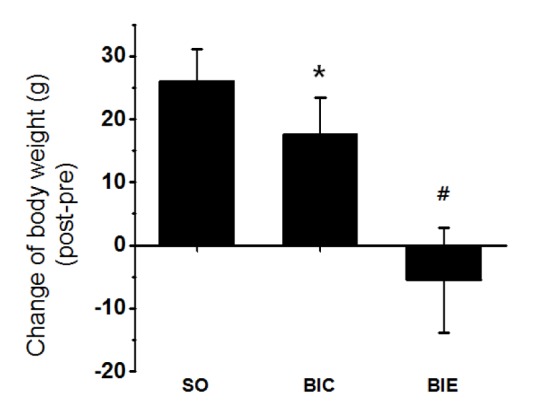
Endurance exercise inhibited elevation of body weight
We’ve checked whether the endurance exercise reduced body weight in atherosclerosis rat model (Fig. 1). 3 weeks of endurance exercise inhibited the elevation of body weight in BIC than SO (p < .05, 26 ± 5.2g vs. 17.6 ± 5.8g), while the body weight was significantly reduced in BIE compared with BIC (p < .05, 17.6 ± 5.8g vs. -5.5 ± 8.3g).
Endurance exercise inhibited balloon-induced neointimal formation
To investigate the effect of endurance exercise on neointimal formation in balloon-injured rat, we’ve analyzed neointimal formation in injured carotid arteries of rats (Fig. 2). Neointimal formation in BIC was significantly higher than SO (p < .05, 1.73 ± 0.35%), but it was significantly recovered in BIE compared with BIC (p < .05, 0.81 ± 0.21%).
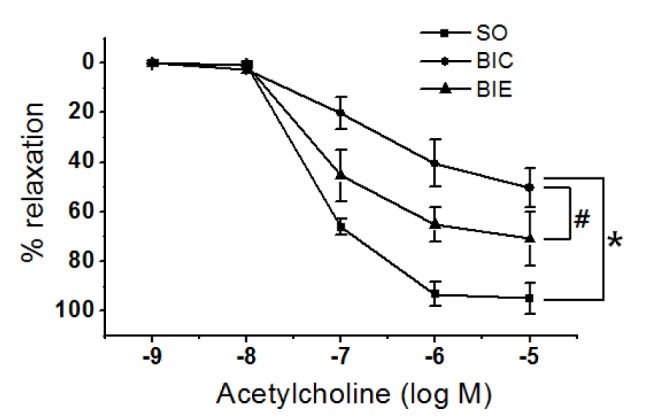
Endurance exercise increased endothelial-dependent relaxation
In order to investigate the effect of endurance exercise on endothelial dependent relaxation in balloon-injured rat, we’ve analyzed endothelial dependent relaxation in injured carotid arteries of rats (Fig. 3). Endothelial-dependent relaxation in BIC was significantly lower than SO (p < .05, 50.5 ± 7.8%, 95.0 ± 6.3%), but it was significantly recovered in BIE (p < .05, 70.91 ± 10.8%) compared with BIC.
Endurance exercise increased FOXOs expression
We investigated FOXOs expression in the injured carotid arteries of rats (Fig. 3). FOXO1 was significantly increased in BIC than SO (p < 0.05, 1.0 ± 0.2 vs. 1.8 ± 0.4), but it was more significantly increased in BIE than BIC (p < .05, 1.8 ± 0.4 vs. 2.52 ± 0.5).
DISCUSSION
Atherosclerosis is the leading cause of death among cardiovascular diseases. Many strategic therapies have been sought to prevent or treat the disease [1,5,21]. One of the well-known strategic methods to prevent or treat of the atherosclerosis is regular exercise [28-30].
It has been reported that regular exercise not only inhibits atherosclerotic lesion in blood vessels but also directly inhibits neointimal formation [28-30]. We’ve also confirmed in this study that endurance exercise actually inhibited neointimal formation in balloon-injured rats (Fig. 2). Body weight was also significantly reduced in a rat group that had endurance exercise (Fig. 1). These results suggest that moderate endurance exercise is the appropriate intensity of the exercise to effectively inhibit neointiaml formation and may be directly evidence to inhibit atherosclerosis.
Chronic endothelial injury or endothelial dysfunction causes atherosclerosis. However, regular exercise induces fast recovery of damaged endothelioid cells and enhances endothelial-dependent relaxation which is protective against eNOS-NO dysfunction [33]. The effect of endurance exercise on endothelial-dependent relaxation was also investigated in this study (Fig. 3). Endothelial-dependent relaxation in BIC was significantly lower than SO, suggesting endothelial dysfunction. But the study results indicated that dysfunction of endothelial cells in atherosclerosis can be effectively improved by endurance exercise. Blood flow-derived vascular relaxation by the exercise is reportedly caused by increase in shear stress which means endurance exercise can enhance endothelial-dependent relaxation, resulting in improvement of atherosclerosis.
However, molecular mechanism of exercise for the prevention or treatment of the atherosclerosis has not been studied widely. Although recent studies have reported increase of eNOS-NO [28,29] and inhibition of SIRT-1 activation by exercise [7,9], not many studies have been done on the role of exercise in decreasing FOXO proteins which play an essential role for development of atherosclerosis [21,22].
Many studies have focused on FOXO transcription factors, one of the main downstream mediators of PI3K-AKT signal transduction pathway [21-23]. FOXO proteins are important in development of atherosclerosis because shear stress induces activation of AKT, eNOS and AMPK (AMP activated protein kinase) [33]. FOXO proteins increase Catalase and MnSOD in both endothelial cells and vascular smooth muscle cells, playing an important role in removing ROS [23]. Especially, increase of FOXO proteins reportedly inhibits migration of vascular smooth muscle cells [24] and neointima formation [21,22,24].
Thus, this study investigated whether endurance exercise increased the expression of FOXO proteins in atherosclerotic blood vessels (Fig. 4). The results showed that the exercise effectively increase expression of FOXO1 and FOXO3a, suggesting that endurance exercise can increase expression of FOXO1 and FOXO3a proteins to result in improvement of atherosclerosis.

Endurance exercise increased expression of FOXO1 and FOXO3a in balloon-induced common carotid arteries of rat. SO, Sham-operated control; BIC, Balloon-injured control; BIE, Balloon-injured and exercise. Each data showed mean ± S.E.M. * p < .05 vs. SO; #p < .05 vs. BIC.
Since FOXO proteins are associated with very complicated signal transduction pathways, its role in development of atherosclerosis is not clear. FOXO proteins generally exist in nucleus but move to protoplasm and lose their function when they are phosphorylated by external stimulus such as growth factors or insulin [23]. Since phosphorylation of FOXO proteins means inactivation of the protein, it is important to investigate if exercise leads to phosphorylation of FOXO proteins. However, this study could not look into this subject, suggesting a need for more studies not only on expression of FOXO proteins but also on phosphorylation of FOXO proteins.
In conclusion, these results suggest that endurance exercise may inhibit neointimal formation and endothelial-dependent relaxation via FOXO1 and FOXO3a expression in balloon-induced atherosclerosis rat model.
Acknowledgements
This work was supported by the National Research Foundation of Korea Grant funded by the Korean Government (NRF-2011-332-G00088).
All experiments were approved by the Animal Care and Use Committee at the Chungnam National University (CNU-00052).