 |
 |
- Search
| Phys Act Nutr > Volume 26(4); 2022 > Article |
|
Abstract
[Purpose]
Although several physiological roles of lactate have been revealed in the last decades, its effects on energy metabolism and substrate oxidation remain unknown. Therefore, we investigated the effects of lactate on the energy metabolism of resting rats.
[Methods]
Male rats were divided into control (Con; distilled water), caffeine (Caf; 10 mg/kg), L-lactate (Lac; 2 g/kg), and lactate-plus-caffeine (Lac+Caf; 2 g/ kg + 10 mg) groups. Following oral administration of supplements, resting energy expenditure (study 1), biochemical blood parameters, and mRNA expression involved in energy metabolism in the soleus muscle were measured at different time points within 120 minutes of administration (study 2). Moreover, glycogen level and Pyruvate dehydrogenase (PDH) activity were measured.
[Results]
Groups did not differ in total energy expenditure throughout the 6 hour post-treatment evaluation. Within the first 4 hours, the Lac and Lac+Caf groups showed higher fat oxidation rates than the Con group (p<0.05). Lactate treatment decreased blood free fatty acid levels (p<0.05) and increased the mRNA expression of fatty acid translocase (FAT/CD36) (p<0.05) and peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) (p<0.05) in the skeletal muscle. Hepatic glycogen level in the Lac+Caf group was significantly increased (p<0.05). Moreover, after 30 and 120 minutes, PDH activity was significantly higher in lactate-supplemented groups compared to Con group (p<0.05).
Lactate has been regarded as a waste product of anaerobic glycolysis (e.g., during high-intensity exercise), and because of its acidic nature, has often been viewed as a potential cause of muscle fatigue during exercise [1,2]. However, lactate production occurs naturally regardless of oxygen concentration in our body. Moreover, the lactate acidosis paradigm has been drastically reconsidered, as it is currently known that lactate is not responsible for acidosis. Instead, acidosis associated with exercise has been argued to be induced by increased H+ production from ATP hydrolysis [3]. Based on the lactate shuttle theory, lactate can be produced and transported to adjacent organs, where it is oxidized. During exercise of any intensity, the energy for muscular contraction is also provided by lactate.
Motivated by the novel physiological roles of lactate, researchers have investigated the effects of exogenous lactate administration on muscle. For example, lactate administration upregulates peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) mRNA, which is related to mitochondrial biogenesis in the muscle [4]. Moreover, a cell-based approach revealed that lactate supplementation increases satellite cell activity and anabolic signals in C2C12 cells [5]. In rats, administration of a lactate-based supplement associated with low-intensity exercise elevates the number of myonuclei, indicating that lactate effectively promotes the development of muscle mass [5]. Furthermore, lactate is used as fuel much faster and to a greater extent than glucose, when ingested in sports drinks [6]. Nevertheless, it is still unknown how exogenous lactate is metabolized with time and how it affects energy substrate utilization. Notably, these previous studies used lactate-based supplements, which actually contain lactate and caffeine. Hashimoto et al. [7] reported that sodium lactate and caffeine treatment followed by voluntary wheel running for 5 weeks lowered the scapular fat weight relative to that of the exercise group, along with lower body weight than that of the sedentary group. Concurrently, proteins, including comparative gene identification (CGI)-58 and hormone sensitive lipase (HSL), were higher in the lactate- and caffeine-treated groups than in the sedentary group. However, previous studies have not elucidated the metabolic effects of lactate or caffeine. Therefore, an investigation of the metabolic effects of lactate alone and in combination with other substances is needed [8]. Moreover, our laboratory previously studied the effect of lactate administration in terms of glycogen synthesis and demonstrated that glycogen synthesis enzymes such as pyruvate carboxylase and glycogen synthase 2, increased following treatment with the same dosage of lactate [9]. In this context, the glycogen levels and specific mechanism based upon previous findings require further investigation.
The purpose of this study was to examine the effect of lactate on energy metabolism and energy substrate utilization in rats. This effect was compared to that of caffeine and a mixture of lactate and caffeine, as caffeine is commonly used as a positive control in these types of studies due to the promotion of fat oxidation, energy expenditure [10-13], and exogenous carbohydrate oxidation during exercise [14]. We hypothesized that lactate can significantly increase fat oxidation and exert a synergistic effect with caffeine, which might enhance fat metabolism-related mRNAs. Thus, we hypothesized that lactate administration can enhance hepatic glycogen concentration.
Male Sprague-Dawley rats (7 weeks old) were housed in standard plastic cages under controlled relative humidity (50 %) and temperature (23 ± 1°C) conditions, with alternating 12 hours of light (07:00-19:00) and dark (19:00-07:00) cycles [15,16]. The experiments were performed during the 12 hours light cycle. Rats were fed ad libitum with a commercial normal diet. This study was conducted in accordance with the ethical guidelines of the Konkuk Institutional Animal Care and Use Committee (KU16018, KU18137).
We performed two studies referred to as study 1 and study 2 (n = 32 per study; Figure 1). In study 1, rats were divided into control (Con; distilled water, n = 8), caffeine (Caf; 10 mg/kg, n = 8), L-lactate (Lac; 2 g/kg, n = 8), and lactate-plus-caffeine (Lac+Caf; 10 mg + 2 g/kg, n = 8) groups for both studies. Rats were starved for 2 hours prior to administration. The resting metabolic rate (RMR) was recorded using indirect calorimetry for 6 hours after the treatment. In study 2, the same number of rats were assigned to each group such as control (Con; distilled water, n = 8), caffeine (Caf; 10 mg/kg, n = 8), L-lactate (Lac; 2 g/kg, n = 8), and lactate-plus-caffeine (Lac+Caf; 2 g/kg + 10 mg, n = 8). Rats were starved for 2 hours prior to administration. Then rats were euthanized via cervical vertebral dislocation at different time intervals (0, 30, 60, and 120 minutes) after oral administration of treatments. Subsequently, skeletal muscle (soleus muscle), liver, and blood samples were obtained, frozen in liquid nitrogen, and stored at -80 °C until analysis of mRNA levels and biochemical parameters. The soleus muscle is selected for mRNA analysis due to its oxidative characteristics. The dosages for treatments were based on those used in previous studies [17,18], and treatments were orally administered. In a previous study, 3 g/kg of lactate was intraperitoneally injected to set the blood lactate levels at approximately 20 mmol/L, which are similar to those attained upon maximal exercise 18 . However, this study aimed to examine the basic effect of lactate on metabolism. Therefore, we selected a lower stimulant dose and a previously reported administration method.
To examine the effect of lactate on energy substrate utilization in the whole body, we measured the RMR for 6 hours after treatment using an open circuit calorimetric chamber (model RL-600, Alco System, Chiba, Japan). This equipment is known for its accuracy and is widely used for calculating gas exchange data [19,20]. We measured the RMR as previously described [21-23].
Blood samples were obtained from the pulmonary artery using a syringe and stored in EDTA tubes for centrifugation. The resultant plasma samples were then analyzed for lactate, glucose, free fatty acids (FFA), and glycerol levels using commercial kits (Lactate Colorimetric/Fluorometric Assay kit, Biovision; Glucose Colorimetric Detection Kit, Arbor Assays; Free Fatty Acid Assay Kit. Cell biolabs; Glycerol Colorimetric Assay kit, Cayman chemical). For mRNA analyses, we extracted RNA from the soleus muscle and analyzed it by reverse transcriptase PCR method. The soleus muscle was selected for analysis as we did not have any other treatment except for supplementation. Additionally, we believed that the soleus muscle would be the most appropriate tissue to study the metabolic changes. Commercial kits were used for cDNA synthesis and amplification (amfiRivert cDNA Synthesis Platinum Mater Mix, R5600, Gendepot, USA, amfiEco Taq DNA Polymerase, P0701, Gendepot). We measured the mRNA expression of glyceraldehyde 3-phosphate dehydrogenase (GAPDH), fatty acid translocase (FAT/CD36), carnitine palmitoyltransferase 1b (CPT1b), PGC-1α, and pyruvate dehydrogenase kinase 4 (PDK4). The expression levels of these genes were normalized to that of GAPDH. The sequences of primers used for PCR are listed in Table 1.
Glycogen levels were measured from 30 mg of gastrocnemius muscle and 30 mg of liver, respectively. Using the amyloglucosidase method [24], we added 500 uL of 2 N HCL to these specimens and incubated the mixtures on a heating block for 2 hours at 96 °C. During the 2 hours of incubation, the tubes were tapped every 30 minutes to ensure proper digestion. After 2 hours of incubation, 1500 uL of 0.67 M NaOH was added and centrifuged for 5 minutes at 5000 x g, at 4 °C. The supernatant was mixed with reaction buffer and incubated for 30 minutes at room temperature. The glucose content was analyzed using a spectrophotometer (Thermo Fisher scientific, USA) at 340 nm.
PDH activity was measured from liver tissue samples using the PDH Activity Colorimetric Assay Kit (Biovision, Mountain View, CA, USA). According to the instructions, we used BioVision’s Mitochondria Isolation Kit (K288-50) to isolate mitochondria from the tissue. The PDH activity was determined using 10 mg of liver sample. The colorimetric measurement of the kinetic reduction of NAD+ to NADH was conducted for 60 minutes at 450 nm.
Data are presented as mean ± standard error (SE). In study 1, statistical significance was evaluated using two-way repeated measures analysis of variance (ANOVA) for respiratory gas exchange data and one-way ANOVA for the 2 hours sum of respiratory gas exchange data. In study 2, one-way ANOVA was used to determine the changes in mRNA levels and blood parameter concentrations, and Bonferroni post-hoc analysis was conducted if significance was reached. Differences were considered significant at P < 0.05. Differences between the groups for the analysis of glycogen were determined by independent sample t-test. For statistical analysis of PDH activity, we used two-way repeated measures analysis of variance (ANOVA), and one-way ANOVA between the groups in the same time period. P < 0.05 was considered statistically significant. PDH
In study 1, we measured the RMR for 6 hours in each group, whereby we calculated energy expenditure, fat and carbohydrate oxidation, and respiratory exchange ratio. Energy expenditure did not differ significantly among groups throughout the measurement period (Figure 2a). Moreover, the accumulated energy expenditure within 2 hours intervals did not differ significantly (Figure 2b).
The rates of fat oxidation were higher in the Lac and Lac+Caf groups than in the Con group throughout the 6 hours measurement (Figure 3a). Furthermore, animals in the Lac and Lac+Caf groups showed significantly higher fat oxidation in the first 4 hours post-administration than those in the Con group (p<0.05) (Figure 3b). Interestingly, when the global rate of fat oxidation was analyzed in relation to the total time of measurement (6 h), only the Lac+Caf group presented higher fat oxidation rates than those of the Con group (Figure 4). As expected, an opposite trend was observed for carbohydrate oxidation (Figure 5a), which was lower in the first 2 hours post-administration in the Lac+Caf group than in the Con group (p<0.05) (Figure 5b).
Plasma lactate levels were higher in the Lac, Caf, and Lac+Caf groups than in the Con group during the first 60 minutes after treatments (p<0.05) (Table 2). At 120 minute, there were no significant differences between groups. Moreover, no significant changes were observed in plasma glucose levels at any sampling time (Table 2). In contrast, we found significant differences in FFA levels between groups at all sampling times (p<0.05) (Table 2). Lactate administration, as either Lac or Lac+Caf, significantly lowered FFA levels relative to those of the Con group at 30 minute (p<0.05). In that regard, the co-administration of caffeine and lactate resulted in lower levels of FFA than those in animals treated with lactate alone. This pattern changed at 60 minute, when FFA levels were higher in the Lac and Lac+Caf groups than in the Con group (p<0.05), and higher levels of FFA were noted in caffeine-treated animals (Caf and Lac+Caf) than in animals that received only lactate (p<0.05). After 120 minutes, the only significant difference in FFA levels was between the Con and Lac groups, wherein animals treated with lactate had lower FFA concentrations than Con animals (p<0.05). In the case of glycerol, the Caf group showed significantly higher plasma levels than the Con and Lac groups 30 minutes after the treatments (p<0.05) (Table 2). Furthermore, the Caf group presented the highest glycerol level at 60 minute (p<0.05), whereas no changes were observed between groups at 120 minute.
The transcript levels of FAT/CD36 did not differ significantly during the first 30 minutes (Figure 6a). However, at 60 minute, the Lac group had significantly higher FAT/CD36 mRNA levels than the Lac+Caf group (p<0.05) (Figure 6a). Moreover, the Lac group presented higher FAT/CD36 mRNA expression than the Con and Caf groups after 120 minutes (p<0.05) (Figure 6a). No changes were observed in CPT1b mRNA levels among groups (Figure 6b). In the case of PGC-1α expression, mRNA levels were higher in the Lac group than in the Caf and Lac+Caf groups at 30 minute (p<0.05) (Figure 6c). The same trend was observed for PDK4 mRNA, whose levels were higher in the Lac group than in the Caf and Lac+Caf groups at 30 minute (p<0.05) (Figure 6d).
We examined the glycogen concentration following treatment with supplements. Lac and Lac+Caf were selected based on previous results. Either of the treatments was administered to each group to examine the changes in glycogen levels. The glycogen concentration did not show significant changes following lactate administration (Figure 7-a,c). However, following treatment with Lac+Caf, the concentration of glycogen was significantly increased (p<0.05) (Figure 7-d).
The PDH activity level was measured following the administration of each supplement at timepoint (0, 30, 60, and 120 minutes) (Figure 8). Interestingly, the Lac+Caf and Lac group maintained significantly higher PDH activity for the entire 120 minutes following administration compared to the Con group (p<0.05). The Lac and Lac+Caf groups showed significantly higher levels compared to Caf at the 30- and 120-minute time point as well (p<0.05)-highlighting the effect of lactate-based supplements on PDH activity.
In this study, we aimed to investigate the effect of exogenous lactate and a lactate-based supplement on energy metabolism and energy substrate utilization in resting rats. We assessed whole-body energy metabolism in study 1 and further conducted gene expression analysis and blood parameter assessment in study 2. The parameters for gene expression and blood analysis were determined based on the results of study 1.
The most interesting findings were those related to energy metabolism in the whole body of animals. We could not detect significant differences in total energy expenditure rates between the groups. The lack of changes may be related to our experimental design of an acute single bout of supplement, which may not be enough to affect whole energy expenditure. Nevertheless, this protocol was enough to significantly affect fat oxidation. We found that fat oxi-dation increased in response to the acute Lac and Lac+Caf treatments in the first 4 hours after administration. Although not statistically significant, fat oxidation rates in the Lac and Lac+Caf groups tended to be higher than those in animals that received caffeine alone in the initial 4 h. These results are concurrent with previous findings on the combinatorial effects of lactate and caffeine. Chronic caffeine intake is known to reduce body fat levels [7]. Therefore, we could not determine whether fat reduction after combinatorial caffeine and lactate treatment resulted from the synergic effect of both supplements or the individual effects of caffeine and lactate. Moreover, in a previous study, the ingestion of caffeine increased the metabolic rate by 0.2 kJ/min and this increase lasted for 3 hours in healthy male volunteers [12]. Similar results were also reported in resting rats, where their O2 uptake was significantly higher (P < 0.05) 2 hours after the administration of 10 mg of caffeine than that of the control [13]. In our study, we detected differences in fat oxidation for a longer period (4 h) after the acute administration of lactate and lactate-plus-caffeine. Moreover, when the fat oxidation data during the 6 hours were combined, we found that the Lac+Caf group had significantly higher fat oxidation rate than the Con group. Considering that caffeine alone did not cause significant changes in fat oxidation, lactate alone can be regarded as a remarkable supplement that induces fat oxidation in vivo. Indeed, mice treated for 14 days with lactate injections (2 g/kg) showed 4 % weight loss [25]. Overall, our results indicate that not only lactate, but also lactate-plus-caffeine, may have positive effects on fat oxidation. The mechanisms by which lactate and lactate-plus-caffeine exert their effects require elucidation.
To explore the biochemical and molecular mechanisms underlying the effects of exogenous lactate administration, we assessed selected mRNAs in muscle samples and biochemical parameters in blood. Based on our acute experimental design, we determined that mRNA analysis would be more appropriate than protein analysis. We found that two transcripts associated with energy metabolism, FAT/CD36 and PGC-1α, were affected by lactate treatment. FAT/CD36 plays an important role in transporting fatty acids, while PGC-1α is the main regulator of mitochondrial biogenesis. Other functions of PGC-1α include regulation of the metabolic rate [26], control of mitochondrial gene expression [27], and modulation of many genes related to energy homeostasis and mitochondrial biology [28,29]. Our observations are consistent with those of previous studies, in which PGC-1α levels increased in L6 cells (isolated from rat skeletal muscle) after 6 hours of lactate treatment (10 and 20 mM) [4,30]. Interestingly, lactate, which is regarded as a harmful byproduct of glycolysis, induces the expression of PGC-1α. Lactate has been recently reported to be strongly associated with transforming growth factor-beta 2 (TGF-β2), which improves glucose tolerance and insulin sensitivity, increases fatty acid uptake and oxidation, and stimulates glucose uptake in the skeletal muscle, heart, and brown adipose tissue [31]. These effects of TGF-β2 may have stimulated fat metabolism herein. Similarly, numerous proteins enhancing fat metabolism may have been stimulated upon lactate treatment. Therefore, the expression of other proteins needs to be analyzed after chronic lactate administration at an optimized dose. Previous studies have used protocols involving the injection of lactate into animals or the incubation of cells with lactate. However, we administered lactate orally, which is a more convenient and less invasive route of administration. Nevertheless, our results are consistent with those of studies using other routes, indicating that this approach may be used to further investigate the role of exogenous lactate, including its specific mechanisms and effects of chronic treatment. Furthermore, the analysis of protein levels may also shed some light on the underlying mechanism of action. Additionally, our understanding of the results of blood parameter analysis is limited. Caffeine increased fat oxidation-enhanced lipolysis, such that FFA levels were increased in 60 minutes, while lactate did not cause drastic alteration in FFA levels compared to Con treatment. However, gas exchange analysis showed that lactate increased fat oxidation in the whole body. Therefore, the effect of lactate on blood and whole-body parameters requires more supportive data from studies involving chronic lactate treatment to elucidate the underlying mechanism.
Considering the other applications of lactate, based on respiratory gas exchange data and mRNA analysis, we could detect an imbalance between carbohydrate and fat oxidation, in which carbohydrate oxidation was inhibited and fat oxidation was enhanced. These results were confirmed via microarray analysis (data not shown) by the presence of several enzymes such as phosphoenolpyruvate carboxykinase 1 (PCK1) and acetyl-CoA acetyltransferase 2 (acat2) which has a role in carbohydrate and lipid metabolism, respectively. Moreover, we demonstrated an increase in hepatic glycogen level via a single bout of Lac+Caf administration. Likewise, in mice, the chronic administration of 2.5 mg/g lactate for 3 weeks after a bout of treadmill exercise increased glycogen concentration in the white muscle when compared to mice treated with saline [32]. Moreover, lactate incubation with C2C12 cells (mouse muscle cells) increased the follistatin protein level and phosphorylation of P70S6K, which are anabolic signals and critical regulators of exercise-induced muscle protein synthesis and hypertrophy [5]. These observations imply that post-exercise lactate administration could be effectively used to optimize muscular anabolic signals and glycogen restoration, which may have important implications in the training context (e.g., for athletes). Furthermore, the PDH activity was enhanced following the administration of lactate-based supplements. In this regard, lactate enhances both glycogen synthesis and glycolysis regulated by PDH activity. From previous research [33,34], upregulated PDHa activity by exercise was examined concomitant with elevated plasma FFA levels. Moreover, elevated FFA levels are also associated with enhanced PDK 4 expression [35,36]. In the current study, the whole-body gas results confirmed the increase in fat oxidation with the administration of Lac+Caf. Therefore, based on the previous studies, metabolic changes in enhancing fat oxidation may induce PDK4 and PDH activity similar to when exposed to exercise, resulting in increased hepatic glycogen concentration. Based on these findings, the research for determining optimizing timing for lactate supplementation on sparing circulating carbohydrate and glycogen reserves. Moreover, further research is required to study the effect of Lac+Caf with different component ratios on glycogenesis and fat oxidation. This may potentially improve the management of individualized supplementation on individuals’ purpose of either glycogen synthesis or fat oxidation or different component ratio based on supplementation timings. Lastly, another possible application of lactate is for the treatment of sedentary people. Albeit no significance in energy expenditure, we demonstrated a single bout of lactate administration enhanced fat oxidation. Therefore, our results suggest that chronic lactate intake potentially enhances fat oxidation, thus potentially leading to body fat loss, even in sedentary individuals. Furthermore, our findings indicate that the combination of low-intensity exercise, which is not burdensome to sedentary individuals, and regular dietary control with lactate treatment could exert more significant effects on weight reduction.
Of note, as the purpose of our study was to examine the fundamental effect of lactate supplementation and lactate-based supplements compared to that of control and caffeine treatment, the design of our study involved an acute treatment. Moreover, we considered the practical future application of lactate and decided to administer the supplements orally to mimic the eventual administration route in humans (e.g., drinkable supplements). Therefore, even though we considered the changes among groups in whole-body parameters with P < 0.05 as significant, it is possible that the treatments in the present study were not enough to affect the gene expression and blood parameters.
In conclusion, we found that exogenous lactate orally administered to resting rats increased their fat oxidation rates. The induction of fat oxidation was associated with changes in mRNA levels of key genes involved in lipid metabolism and circulating levels of FFA. Lactate treatment showed remarkable effect on the induction of fat oxidation, thus providing novel insights into the metabolic effects of lactate treatment. Moreover, when ingested with caffeine, lactate also showed sparing effect of glycogen which may elicit huge advantages in athletes. Further research, including the assessment of long periods of treatment, may reveal additional details on the specific mechanisms underlying the metabolic effects of exogenous lactate.
Our current study has a limitation. As lactate administration or infusion is known to induce alkalosis which inhibits carbon dioxide ventilation [37-40], the respiratory exchange ratio could have been misrepresented. Thus, the isotope method could be considered to revise this calculation. However, in the current study, there was no significant difference within groups in terms of oxygen uptake and carbon dioxide production (data not shown). Moreover, the mRNA results show some metabolic changes to be favorable to fat oxidation. Therefore, from the current study—besides alkalosis— lactate administration seems to enhance fat oxidation in the whole body.
Acknowledgments
Choongsung Yoo and Jisu Kim contributed equally to this work. The authors would like to show their gratitude to the Exercise Nutrition Laboratory at Konkuk University for their cooperation in this study. This work was supported by the Ministry of Education of the Republic of Korea and the National Research Foundation of Korea (NRF- 2019S1A5A8033825). This research was supported by the KU Research Professor Program of Konkuk University.
Figure 1.
Study design. In study 1, the resting metabolic rate was measured following the administration of lactate, caffeine, or both. In study 2, blood, muscle, and liver samples were collected at different time intervals for the analysis of mRNA and blood parameters.
Figure 2.
Effects of oral administration of lactate, caffeine, and lactate- plus-caffeine on energy expenditure. (a) Energy expenditure. Time, 0.000; Group, 0.724; Interaction, 0.149. (b) Accumulated energy expenditure. Experimental groups were as follows: control (Con), lactate (Lac), caffeine (Caf), and caffeine plus lactate (Lac+- Caf). Values are presented as mean ± SE (n = 8).
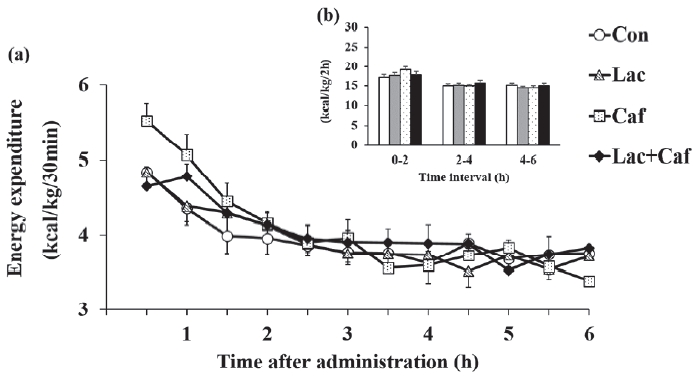
Figure 3.
Effects of oral administration of lactate, caffeine, and lactate-plus-caffeine on fat oxidation. (a) Fat oxidation rates. Time, 0.000; Group, 0.029; Interaction, 0.000. (b) Accumulated fat oxidation rates. Experimental groups were as follows: control (Con), lactate (Lac), caffeine (Caf), and lactate-plus-caffeine (Lac+Caf). Different letters indicate significant differences between groups (P < 0.05). Values are presented as mean ± SE (n = 8).
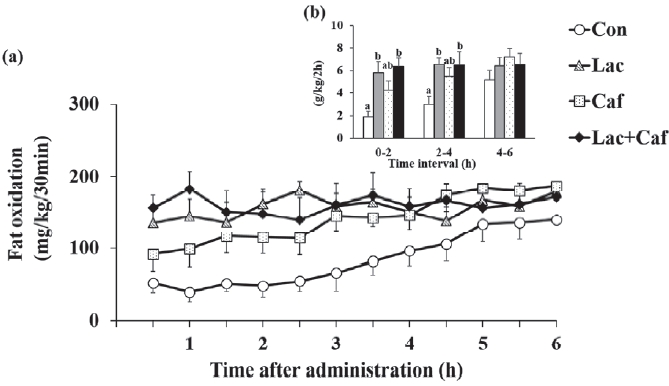
Figure 4.
Effects of oral administration of lactate, caffeine, and lactate-plus-caffeine on fat oxidation rates. Experimental groups were as follows: control (Con), lactate (Lac), caffeine (Caf), and lactate-plus-caffeine (Lac+Caf). Different letters indicate significant differences between groups (P < 0.05). Values are presented as mean ± SE (n = 8).
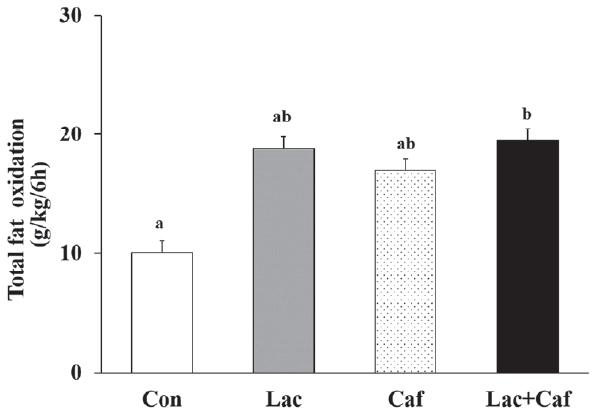
Figure 5.
Effect of oral administration of lactate, caffeine, and lactate plus caffeine on carbohydrate oxidation rate. (a) Carbohydrate oxidation rates. Time, 0.000; Group, 0.078; Interaction, 0.000. (b) Accumulated carbohydrate oxidation rates. Experimental groups were as follows: control (Con), lactate (Lac), caffeine (Caf), and lactate-plus-caffeine (Lac+Caf). Different letters indicate significant differences between groups (P < 0.05). Values are presented as mean ± SE (n = 8).

Figure 6.
Effect of orally administered lactate and lactate co-ingested with caffeine on the expression of genes related to fat metabolism. (a) Fatty acid translocase (FAT/CD36). (b) Carnitine palmitoyltransferase 1b (CPT1b). (c) Peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α). (d) Pyruvate dehydrogenase kinase 4 (PDK4). Experimental groups were as follows: control (Con), lactate (Lac), caffeine (Caf), and lactate-plus-caffeine (Lac+Caf). Representative blots are shown on the top of each panel. Different letters indicate significant differences between groups (P < 0.05). Values are presented as mean ± SE (n = 8).

Figure 7.
Glycogen concentration analysis. (a), (b) The glycogen concentration in muscle following administration of lactate and lactate with caffeine respectively. (c), (d) The glycogen concentration in liver following administration of lactate and lactate with caffeine respectively. Different letters indicate significant differences between groups (P < 0.05). Values are presented as mean ± SE.
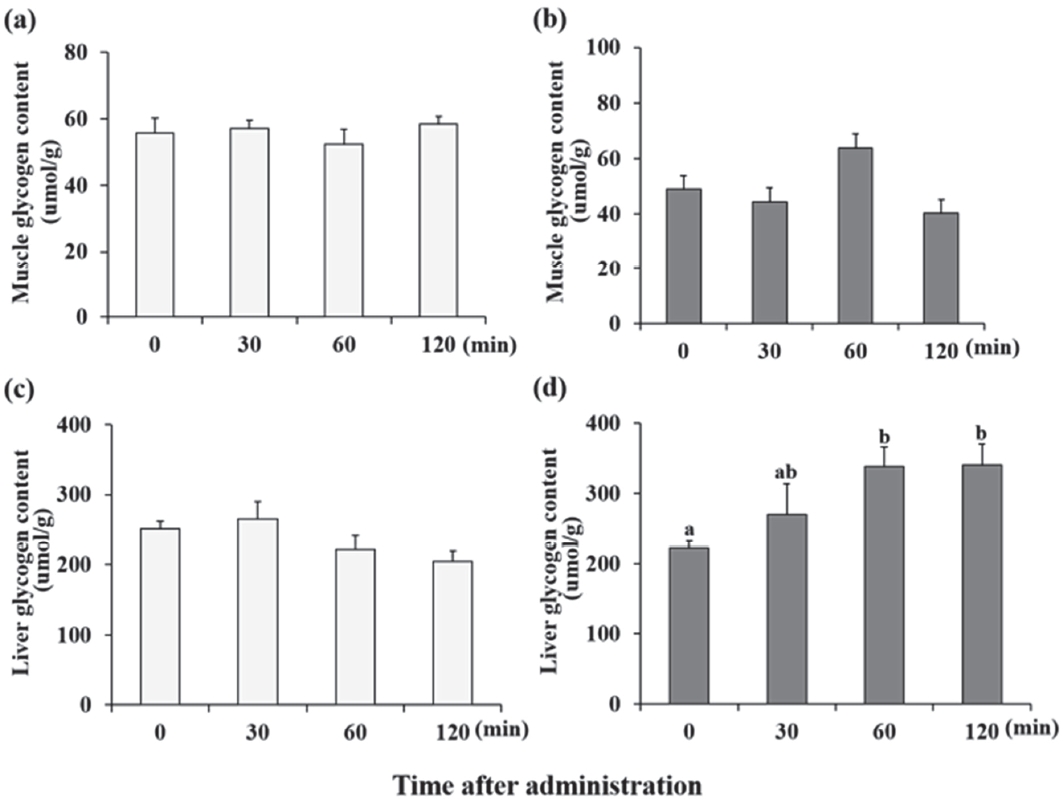
Figure 8.
PDH activity analysis. Experimental groups were as follows: control (Con), lactate (Lac), caffeine (Caf) and lactate- plus-caffeine (Lac+Caf). Different letters indicate significant differences between groups (P < 0.05). Values are presented as mean ± SE (n = 8).
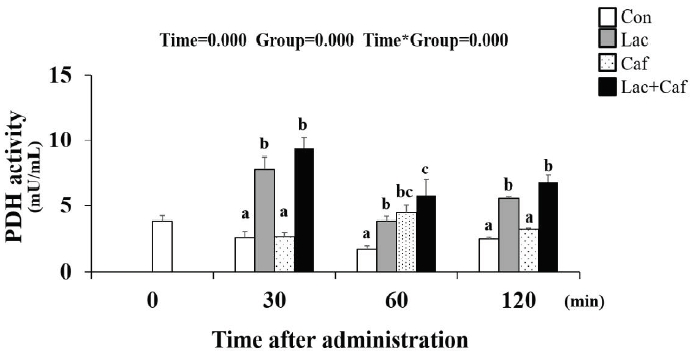
Table 1.
Sequences of PCR primers used in the study.
Table 2.
Key metabolite concentrations in the blood of rats treated with lactate, caffeine, and lactate-plus-caffeine.
Con, rats treated with distilled water; Lac, rats treated with 2 g/kg lactate; Caf, rats treated with 10 mg/kg caffeine; Lac+Caf, rats treated with 10 mg/kg caffeine plus 2 g/kg lactate; FFA, free fatty acids. Values are presented as mean ± SE. Different superscript letters indicate significant differences between groups within the sampling time (P < 0.05).
REFERENCES
1. Farrell PA, Wilmore JH, Coyle EF, Billing JE, Costill DL. Plasma lactate accumulation and distance running performance. Med Sci Sports 1979;11:338-44.


2. Donovan CM, Pagliassotti MJ. Enhanced efficiency of lactate removal after endurance training. J Appl Physiol (1985) 1990;68:1053-8.


3. Robergs RA, Ghiasvand F, Parker D. Biochemistry of exercise-induced metabolic acidosis. Am J Physiol Regul Integr Comp Physiol 2004;287:R502-16.


4. Kitaoka Y, Takeda K, Tamura Y, Hatta H. Lactate administration increases mRNA expression of PGC-1α and UCP3 in mouse skeletal muscle. Appl Physiol Nutr Metab 2016;41:695-8.


5. Oishi Y, Tsukamoto H, Yokokawa T, Hirotsu K, Shimazu M, Uchida K, Tomi H, Higashida K, Iwanaka N, Hashimoto T. Mixed lactate and caffeine compound increases satellite cell activity and anabolic signals for muscle hypertrophy. J Appl Physiol (1985) 2015;118:742-9.


6. Azevedo JL, Tietz E, Two-Feathers T, Paull J, Chapman K. Lactate, fructose and glucose oxidation profiles in sports drinks and the effect on exercise performance. PLoS One 2007;2:e927.



7. Hashimoto T, Yokokawa T, Narusawa R, Okada Y, Kawaguchi R, Higashida K. A lactate-based compound containing caffeine in addition to voluntary running exercise decreases subcutaneous fat mass and improves glucose metabolism in obese rats. J Funct Foods 2019;56:84-91.

8. Philp A, Macdonald AL, Watt PW. Lactate--a signal coordinating cell and systemic function. J Exp Biol 2005;208:4561-75.



9. Kyun S, Yoo C, Hashimoto T, Tomi H, Teramoto N, Kim J, Lim K. Effects of exogenous lactate administration on fat metabolism and glycogen synthesis factors in rats. Phys Act Nutr 2020;24:1-5.

10. Bellet S, Kershbaum A, Finck EM. Response of free fatty acids to coffee and caffeine. Metabolism 1968;17:702-7.


11. Kim J, Park J, Lim K. Nutrition supplements to stimulate lipolysis: a review in relation to endurance exercise capacity J Nutr Sci Vitaminol (Tokyo). 2016;62:141-61.

12. Koot P, Deurenberg P. Comparison of changes in energy expenditure and body temperatures after caffeine consumption. Ann Nutr Metab 1995;39:135-42.


13. Dulloo AG, Geissler CA, Horton T, Collins A, Miller DS. Normal caffeine consumption: influence on thermogenesis and daily energy expenditure in lean and postobese human volunteers. Am J Clin Nutr 1989;49:44-50.


14. Yeo SE, Jentjens RL, Wallis GA, Jeukendrup AE. Caffeine increases exogenous carbohydrate oxidation during exercise. J Appl Physiol (1985) 2005;99:844-50.


15. Kim J, Lee KP, Beak S, Kang HR, Kim YK, Lim K. Effect of black chokeberry on skeletal muscle damage and neuronal cell death. J Exerc Nutrition Biochem 2019;23:26-31.



16. Park Y, Jang I, Park HY, Kim J, Lim K. Hypoxic exposure can improve blood glycemic control in high-fat diet-induced obese mice. Phys Act Nutr 2020;24:19-23.

17. Kim J, Jeon Y, Hwang H, Suh H, Lim K. Effects of oral caffeine and capsaicin administration on energy expenditure and energy substrates utilization in resting rats. J Exerc Nutr Biochem 2011;15:183-9.

18. Cerda-Kohler H, Henríquez-Olguín C, Casas M, Jensen TE, Llanos P, Jaimovich E. Lactate administration activates the ERK1/2, mTORC1, and AMPK pathways differentially according to skeletal muscle type in mouse. Physiol Rep 2018;6:e13800.




19. Haramizu S, Kawabata F, Masuda Y, Ohnuki K, Watanabe T, Yazawa S, Fushiki T. Capsinoids, non-pungent capsaicin analogs, reduce body fat accumulation without weight rebound unlike dietary restriction in mice. Biosci Biotechnol Biochem 2011;75:95-9.


20. Kim J, Hwang H, Yun HY, Kim B, Lee CH, Suh H, Lim K. Silk peptide intake increases fat oxidation at rest in exercised mice. J Nutr Sci Vitaminol (Tokyo) 2013;59:250-5.


21. Lim KW, Kim JS, Jeon YR, Hwang HJ, Suh HJ. Orignal paper: measurement of resting metabolic rate using metabolic chamber in resting rats. Phys Act Nutr 2011;15:35-40.
22. Chung N, Lim K. Influence of high fat and different types of carbohydrate diet on energy metabolism in growing mice. J Exerc Nutrition Biochem 2019;23:1-12.

23. Hwang H, Kim J, Park J, Yun H, Cheon WK, Kim B, Lee CH, Suh H, Lim K. Red ginseng treatment for two weeks promotes fat metabolism during exercise in mice. Nutrients 2014;6:1874-85.



24. Passonneau JV, Lauderdale VR. A comparison of three methods of glycogen measurement in tissues. Anal Biochem 1974;60:405-12.


25. E L, Lu J, Selfridge JE, Burns JM, Swerdlow RH. Lactate administration reproduces specific brain and liver exercise-related changes. J Neurochem 2013;127:91-100.




26. Finck BN, Kelly DP. PGC-1 coactivators: inducible regulators of energy metabolism in health and disease. J Clin Invest 2006;116:615-22.



27. Scarpulla RC. Metabolic control of mitochondrial biogenesis through the PGC-1 family regulatory network. Biochim Biophys Acta 2011;1813:1269-78.


28. Jäger S, Handschin C, St-Pierre J, Spiegelman BM. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc Natl Acad Sci U S A 2007;104:12017-22.


29. Rohas LM, St-Pierre J, Uldry M, Jäger S, Handschin C, Spiegelman BM. A fundamental system of cellular energy homeostasis regulated by PGC-1alpha. Proc Natl Acad Sci U S A 2007;104:7933-8.


30. Hashimoto T, Hussien R, Oommen S, Gohil K, Brooks GA. Lactate sensitive transcription factor network in L6 cells: activation of MCT1 and mitochondrial biogenesis. FASEB J 2007;21:2602-12.



31. Takahashi H, Alves CRR, Stanford KI, Middelbeek RJW, Nigro P, Ryan RE, Xue R, Sakaguchi M, Lynes MD, So K, Mul JD, Lee MY, Balan E, Pan H, Dreyfuss JM, Hirshman MF, Azhar M, Hannukainen JC, Nuutila P, Kalliokoski KK, Nielsen S, Pedersen BK, Kahn CR, Tseng YH, Goodyear LJ. TGF-β2 is an exercise-induced adipokine that regulates glucose and fatty acid metabolism. Nat Metab 2019;1:291-303.




32. Hoshino D, Hanawa T, Takahashi Y, Masuda H, Kato M, Hatta H. Chronic post-exercise lactate administration with endurance training increases glycogen concentration and monocarboxylate transporter 1 protein in mouse white muscle. J Nutr Sci Vitaminol (Tokyo) 2014;60:413-9.


33. Bradley NS, Heigenhauser GJ, Roy BD, Staples EM, Inglis JG, LeBlanc PJ, Peters SJ. The acute effects of differential dietary fatty acids on human skeletal muscle pyruvate dehydrogenase activity. J Appl Physiol (1985) 2008;104:1-9.


34. St Amand TA, Spriet LL, Jones NL, Heigenhauser GJ. Pyruvate overrides inhibition of PDH during exercise after a low-carbohydrate diet. Am J Physiol Endocrinol Metab 2000;279:E275-83.


35. Bajotto G, Murakami T, Nagasaki M, Tamura T, Tamura N, Harris RA, Shimomura Y, Sato Y. Downregulation of the skeletal muscle pyruvate dehydrogenase complex in the Otsuka Long-Evans Tokushima Fatty rat both before and after the onset of diabetes mellitus. Life Sci 2004;75:2117-30.


36. Schummer CM, Werner U, Tennagels N, Schmoll D, Haschke G, Juretschke HP, Patel MS, Gerl M, Kramer W, Herling AW. Dysregulated pyruvate dehydrogenase complex in Zucker diabetic fatty rats. Am J Physiol Endocrinol Metab 2008;294:E88-96.


37. Ferrannini E, Natali A, Brandi LS, Bonadonna R, De Kreutzemberg SV, DelPrato S, Santoro D. Metabolic and thermogenic effects of lactate infusion in humans. Am J Physiol 1993;265:E504-12.


38. Chioléro R, Mavrocordatos P, Burnier P, Cayeux MC, Schindler C, Jéquier E, Tappy L. Effects of infused sodium acetate, sodium lactate, and sodium beta-hydroxybutyrate on energy expenditure and substrate oxidation rates in lean humans. Am J Clin Nutr 1993;58:608-13.

-
METRICS

-
- 3 Crossref
- Scopus
- 1,987 View
- 55 Download
- Related articles in Phys Act Nutr





